- Corresponding Author:
- S. K. Kashaw
Pharmaceutical Chemistry Division, Department of Pharmaceutical Sciences Dr. H. S. Gour University, Sagar - 470 003, India
E-mail: sushilkashaw@gmail.com
| Date of Submission | 26 September 2006 |
| Date of Revision | 28 July 2007 |
| Date of Acceptance | 26 September 2007 |
| Indian J. Pharm. Sci., 2007, 69 (5): 665-668 |
Abstract
Various novel 2-Imino-3-(4'-carboxamidopyridyl)-5-arylidene-4-thiazolidinones, structurally related to isonicotinic acid hydrazide (isoniazid) were synthesized and evaluated for their antimicrobial and antifungal activities together with their brominated products. Structure of the synthesized compounds was confirmed by means of their IR, 1 H-NMR spectral data and elemental analysis. Investigation of antimicrobial and antifungal activities of compounds was done by liquid dilution method used for the determination of minimum inhibitory concentration. Bacterial strains of Escherichia coli and Staphylococcus aureus and fungal strains of Aspergillus niger and Candida albicans were used to ascertain the activity whereas norfloxacin and amphotericin B was used as the standard positive control for antibacterial and antifungal activities respectively. MIC of the compounds ranged between 6-16 μg/ml and 7-24 μg/ml for antibacterial and antifungal activities respectively. Some of the synthesised compounds showed potent biological activities and were comparable to the standard. Important outcome of the exhaustive screening of all the new candidates in the present experiment was that the introduction of arylidene nuclei at position 5 of the 4-thiazolidinone nucleus significantly improved the biological activity.
Keywords
4-Thiazolidinones, antimicrobial, antifungal, MIC method.
Evaluation of synthetic compounds and natural products for potential antimicrobial activity continues to be an important strategy for the initial identification of new drugs with possible clinical values. With the discovery of 4-thiazolidinones as promising fungitoxic agents1 medicinal chemist’s started working on this nucleus. Their works resulted in bactericidal [2-8] and fungitoxic compounds [9]. Similar studies in our laboratory also demonstrated significant results [10-11]. Isoniazid is a established antitubercular drug and in continuation of our search for potent and novel antibacterial compounds, in this paper we report antibacterial and antifungal activities of a few new chemical entities with 4-thiazolidinone generated from isoniazid.
Materials and Methods
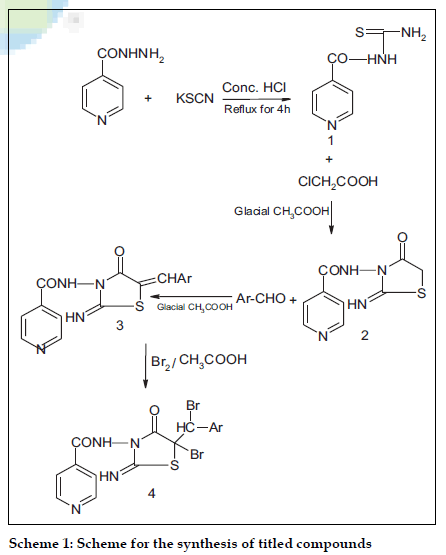
The synthesis of the title compounds is given in scheme 1. Melting points were determined in Toshniwal melting point apparatus using open capillaries method and are uncorrected. The purity of the synthesized compounds was finally ascertained by TLC on silica gel-G plate. The structure of the synthesized compounds was confirmed on the basis of elemental analysis and IR spectral studies on Shimadzu-470 IR in KBr phase. 1H NMR spectra were recorded on Bruker WM-400 NMR spectrophotometer at 200 MHz. Nitrogen analysis was done on Carlo- Erba 1106 instrument.
Isonicotinoyl thiosemicarbazide (1)
A mixture of isonicotinic acid hydrazide (0.1 mol), potassium thiocyanate (0.2 mol), concentrated HCl (8 ml) and water (150 ml) was refluxed for 3 h. The resulting solid that separated was washed with hot water and crystallized from 75% ethanol.
2-Imino-3-(4’-carboxamido pyridyl)-4-thiazolidinone (2)
A mixture of 1 (0.1 mol), monochloroacetic acid (0.1 mol) and anhydrous sodium acetate (0.2 mol) in glacial acetic acid (150 ml) was refluxed for 6 h. After cooling, the reaction mixture was poured on crushed ice and resulting yellow solid that separated was washed with hot water and crystallized from 75% ethanol.
2-Imino-3-(-4’-carboxamido pyridyl)-5-arylidene-4- thiazolidinones (3)
A mixture of 1 (0.01 mol), aromatic aldehyde (0.01 mol) and anhydrous sodium acetate (0.01 M) in glacial acetic acid (25 ml) was refluxed for 5 h. After cooling it was poured on crushed ice and the resultant solid was washed with hot water and crystallized from 70% ethanol. TLC of the compounds was performed on silica gel-G using acetone: DMF (2:1) as solvent system. IR (KBr) cm-1: 3450 (N-H), 1640 (>C=O), 1550,1336 (–N=O nitro), 1500,1580 (-N-H amide), 1460 (–C=N imino), 1240 (–C-N). 1H NMR (in δ ppm) 7.84, 8.70 (pyridyl 4H), 7.77, 8.68 (aromatic,4H), 7.87 (-CONH ), 4.15 (-C=C-H).
Bromination of compounds (4)
A mixture of 3 (0.005 mol) in glacial acetic acid and bromine (0.005 mol) in acetic acid in cold (0-3o) was kept overnight. The crude product obtained was washed with ether and dried. TLC was performed on a silica gel-G plate using benzene:DMF (2:1) as solvent system. IR (KBr) cm-1: 3450 ( N-H), 1640 ( >C=O), 1550,1336 ( –N=O nitro), 1500,1580 (-N-H amide), 1460 (–C=N imino), 1240 (–C-N). 1H NMR (in δ ppm) 7.84,8.75 (pyridyl 4H), 7.97,8.73 (aromatic,4H), 7.91 (-CONH ), 4.07 (-CBr-CH).
Microbiological evaluation
In the present study, liquid dilution method was used for the determination of minimum inhibitory concentration of the synthesized compounds. The MIC was taken as the lowest concentration (highest dilution) without visible growth. Bacterial strains of Escherichia coli (MTCC no. 40) and Staphylococcus aureus (MTCC no. 96) and fungal strains of Aspergillus niger (MTCC no.1344) and Candida albicans (MTCC no. 227) obtained from Institute of Microbial Technology, Chandigarh, India, were used. Nutrient broth was used as the growth medium for the bacteria and Sabouraud’s medium was used for A. niger and Malt yeast medium was used for C. albicans. The incubation was done in electrically heated incubator at 37o for 24 h for bacteria and for fungal cultures it was at 270 for 3 d.
Preparation of solution of synthesized compounds
A stock solution of each compound (1 mg/ml) was prepared in 0.1 N HCl. Required concentrations were prepared by appropriate dilution of stock solution with distilled water. In the same way solution of the standard drug was prepared. In the case of amphotericin B the solution (1 mg/mL) was prepared by dissolving 10 mg of the drug in 1 ml of dimethylsulfoxide and making up the volume to 10 ml with 0.1 N HCl. Further dilutions were then made as required. A loopful of the original lyophilized microbial strain was transferred into the required medium aseptically and incubated at 37o for 48 h for bacteria and at 27o for 3 d for fungi. These were used as stock culture. The sterilization of the culture medium, culture tubes and other materials was done by autoclaving at 15 lb/sq. inch pressure for 20 min.
Determination of minimum inhibitory concentration (MIC)
A set of 8 sterilized test tubes were taken and different solutions were transferred aseptically to each test tubes as per the quantities given in Table 1. Test tubes 6, 7 and 8 were controls. Test tube 6 contained no inhibitor (40% formalin) and confirmed that the culture was viable. Test tube 7 contained neither inhibitor nor organism, which confirmed the sterility of the culture. Test tube 8 contained high concentration of inhibitor but no organisms to detect the precipitation caused by interaction of broth constituents and test compound.
| Test tube no. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Test compound (0.5 mg/ml) | 0.1 ml | 0.2 ml | 0.3 ml | 0.4 ml | 0.5 ml | - | - | 1 ml |
| Inoculum | 0.1 ml | 0.1 ml | 0.1 ml | 0.1 ml | 0.1 ml | 0.1 ml | - | - |
| Broth | 9.8 ml | 9.7 ml | 9.6 ml | 9.5 ml | 9.4 ml | 9.4 ml | 10 ml | 9 ml |
| Final concentration of test compound (µg/ml) | 5 | 10 | 15 | 20 | 25 | - | - | 50 |
| Solvent blank | - | - | - | - | - | 0.5 ml | - | - |
Table 1: Concentrations Of The Compounds Used To Determine Mic.
| CNo. | Ar | Mol. Formula | mp | % Yield | Percentage nitrogen found/ (calculated) | Percentage bromine found/ (calculated) |
|---|---|---|---|---|---|---|
| 3a | p- Nitro phenyl | C16H11N5O4S | 245-47 | 67 | 18.88 (18.96) | - |
| 3b | Phenyl | C16H12N4O2S | 250-52 | 65 | 17.45 (17.27) | - |
| 3c | 3-Methoxy 4-hydroxy phenyl | C17H14N4O4S | 242-44 | 57 | 15.17 (15.12) | - |
| 3d | p-Methyl phenyl | C17H14N4O2S | 256-58 | 65 | 16.47 (16.56) | - |
| 3e | Furfuryl | C14H10N4O3S | 253-55 | 64 | 17.71 (17.82) | - |
| 3f | p-(Trimethyl amino)phenyl | C18H17N5O2S | 258-60 | 68 | 19.02 (19.06) | - |
| 3g | p-Chloro phenyl | C16H11N4O2SCl | 255-57 | 69 | 15.57 (15.61) | - |
| 4a | p- Nitro phenyl | C16H11N5O4S Br2 | 213-15 | 45 | 13.27 (13.23) | 30.10 (30.20) |
| 4b | Phenyl | C16H12N4O2S Br2 | 225-27 | 37 | 11.54 (11.57) | 33.00 (33.12) |
| 4c | 3-Methoxy 4-hydroxy phenyl | C17H14N4O4S Br2 | 236-38 | 41 | 10.53 (10.57) | 30.06 (30.14) |
| 4d | p-Methyl phenyl | C17H14N4O2S Br2 | 245-47 | 44 | 11.20 (11.24) | 32.00 (32.07) |
| 4e | Furfuryl | C14H10N4O3S Br2 | 218-20 | 39 | 11.77 (11.81) | 33.40 (33.71) |
| 4f | p-(Trimethyl amino)phenyl | C18H17N5O2SBr2 | 240-42 | 46 | 13.20 (13.28) | 30.11 (30.31) |
| 4g | p-Chloro phenyl | C16H11N4O2SCl Br2 | 250-52 | 49 | 10.76 (10.81) | 37.40 (37.65) |
Table 2: Physical Data Of The Synthesized Compounds.
| C no. | maxcm-1 | d |
|---|---|---|
| 3a | 3450 (N-H), 1640 (>C=O), 1550,1336 (–N=O nitro), 1500,1580 (-N-H amide), 1460 (–C=N imino), 1240 (–C-N) | 7.84,8.70 (pyridyl 4H), 7.77,8.68 (aromatic,4H),7.87 (-CONH ), 4.15 (-C=C-H ) |
| 3b | 3450 (N-H), 1640 (>C=O), 1500,1580 (-N-H amide), 1460 (–C=N imino), 1240 (–C-N) | 7.90,8.73 (pyridyl 4H), .71(romatic,5H), 7.87 (-CONH), 4.17 (-C=C-H ) |
| 3c | 3450 (N-H), 1640 (>C=O), 1500,1580 (-N-H amide), 1460 (–C=N imino), 1240 (–C-N), 1160 (–C-O phenol), | 7.84,8.70 (pyridyl 4H), 8.68 (aromatic,3H), 7.87 (-CONH), 1050 (–C-O-C methoxy) 4.13 (-C=C-H), 3.95 (-OCH3) |
| 3d | 3450(N-H), 1640(>C=O), 1500,1580 (-N-H amide), 1460 (–C=N imino),1240 (–C-N) | 7.84,8.70 (pyridyl 4H), 8.68 (aromatic,4H), 7.87 (-CONH), 4.13 (-C=C-H ), 2.48 (-CH3) |
| 3e | 3450(N-H), 1640 (>C=O), 1500,1580 (-N-H amide), 1460(–C=N imino),1240 (–C-N, 1140(–C-O furan) | 7.84,8.70 (pyridyl 4H), 7.84 (furyl,3H), 7.87 (-CONH ), 4.14 (-C=C-H ) |
| 3f | 3450 ( N-H), 1640 (>C=O), 1500,1580 (-N-H amide), 1460(–C=N imino), 1240 (–C-N ) | 7.96,8.88 (pyridyl 4H), 7.32,8.68 (aromatic,4H), 7.89 (-CONH), 4.15 (-C=C-H ), 2.42 (-CH3) |
| 3g | 3450 N-H, 1640 >C=O, 1500,1580 (-N-H amide), 1460 (–C=N imino), 1240 (–C-N) | 7.84,8.70 (pyridyl 4H), 8.68 (aromatic,4H), 7.87 (-CONH), 4.14 (-C=C-H ) |
| 4a | 3450 ( N-H), 1640 ( >C=O), 1550,1336 ( –N=O nitro), 1500,1580 (-N-H amide), 1460 (–C=N imino), 1240 (–C-N) | 7.84,8.75 (pyridyl 4H), 7.97,8.73 (aromatic,4H), 7.91 (-CONH ), 4.07 (-CBr-CH ) |
| 4b | 3450 (N-H), 1640 (>C=O), 1500,1580 (-N-H amide), 1460 (–C=N imino), 1240 (–C-N) | 7.99,8.77 (pyridyl 4H), 8.74 (aromatic,5H), 7.39 (-CONH ), 4.07 (-CBr-CH) |
| 4c | 3450 ( N-H), 1640 (>C=O), 1500,1580 (-N-H amide), 1460 (–C=N imino), 1240 (–C-N), 1160 (–C-O phenol), 1050 (–C-O-C methoxy) | 7.84,8.69 (pyridyl 4H), 8.67 (aromatic,3H), 7.87 (-CONH), 4.02 (-CBr-CH) |
| 4d | 3450 (N-H), 1640( >C=O), 1500,1580 (-N-H amide), 1460 (–C=N imino), 1240 ( –C-N) | 7.91,8.72 (pyridyl 4H), 8.56,8.70 (aromatic,4H), 7.39 (-CONH ), 4.06 (-CBr-CH), 2.49 ( methyl) |
| 4e | 3450 (N-H), 1640 (>C=O), 1500,1580 (-N-H amide), 1460 (–C=N imino), 1240 (–C-N, 1140( –C-O furan) | 8.10,8.71 (pyridyl 4H), 5.76 (furyl,3H), 8.0 (-CONH), 4.06 (-CBr-CH) |
| 4f | 3450 ( N-H), 1640 (>C=O), 1500,1580 (-N-H amide), 1460 (–C=N imino ), 1240 (–C-N ) | 7.96,8.88 (pyridyl 4H), 7.32,8.68 (aromatic,4H), 7.89 (-CONH), 4.15 (-C=C-H ), 2.42 (-CH3) |
| 4g | 3450 N-H, 1640 >C=O, 1500,1580 (-N-H amide), 1460 (–C=N imino), 1240 (–C-N ) | 7.93,8.73 (pyridyl 4H), 8.71 (aromatic,4H), 7.90 (-CONH ),4.06 (-CBr-CH) |
Table 3: Spectral Data Of The Synthesized Compounds (3a-F And 4a-F).
| C. No. | Bactericidal (µg/ml) | Fungicidal (µg/ml) | ||
|---|---|---|---|---|
| E. coli | S. aureus | A. niger | C. albicans | |
| 2 | 14 | 16 | 22 | 24 |
| 3a | 8 | 8 | 13 | 15 |
| 3b | 12 | 13 | 18 | 20 |
| 3c | 10 | 12 | 16 | 19 |
| 3d | 13 | 14 | 19 | 20 |
| 3e | 12 | 13 | 20 | 22 |
| 3f | 9 | 12 | 17 | 20 |
| 3g | 6 | 7 | 12 | 14 |
| 4a | 6 | 7 | 7 | 9 |
| 4b | 13 | 12 | 14 | 17 |
| 4c | 10 | 10 | 10 | 14 |
| 4d | 12 | 13 | 13 | 16 |
| 4e | 13 | 14 | 15 | 18 |
| 4f | 8 | 12 | 12 | 14 |
| 4g | 7 | 8 | 8 | 10 |
| Norfloxacin | 2 | 2 | - | - |
| AmphotericinB | - | - | 2 | 2 |
Table 4: Antibacterial And Antifungal Activities Of The Synthesized Compounds.
All the test tubes were incubated for the period as mentioned above and examined for growth of the test organism. The MIC of the test compound was between the lowest concentration inhibiting growth and the highest concentration allowing growth. These two concentrations for each compound were noted. The exact MIC of the each compound was determined by repeating the experiment, using a range of concentrations between these two concentrations.
Results and Discussion
The percentage yield of the compounds, together with mp and elemental (nitrogen and bromine) analyses are reported in Table 2. The structure of the compound (1) was confirmed on the basis of mp, IR and 1H NMR spectra. Compound (2) showed adsorption bands at 3450 cm–1, 1580, 1500 cm–1 ( –NH), 1640 cm–1 (>C=O), 1460 (–C=N of imino group), 1240 (–C-N), and 683 (–C-S stretching). The 1H NMR spectral studies show chemical shift at δ 7.84, 8.70 ppm (pyridyl) 7.87 (-CONH) and 3.45 (-CH2-). Compound (3) showed spectral data as compound (2) to indicate the presence of the 4-thiazolidinone nuclei. Other spectral data specific for the groups substituted or added to the nucleus are given in Table 3. A perusal of Table 4 shows that the MIC of the synthesised compounds ranged between 6-16 µg/mL and 7- 24 µg/mL for bactericidal and fungicidal activity, respectively. A closer look at the Table 4 shows that the introduction of a arylidene nuclei at position 5 of the 4–thiazolidinone nucleus improved the bactericidal activities in certain cases namely 3g, 3a and 3f as against 2. Bromination of the compounds has resulted into 4 with practically no change in their bactericidal potency. As in the case of bactericidal activity there has been improvement in the fungicidal activity of the compounds with arylidene nucleus at position 5 of the nucleus. In this case too the most active compounds are 3a and 3g. However bromination gave some improvement in their fungicidal effect. Most active compounds are 4a and 4g. Another important feature which we observe is that the compounds showing good cidal effects were having either p-NO2 or p-Cl in the phenyl ring group at position 5.
Acknowledgements
The authors thank the RSIC, CDRI Lucknow and Sophisticated Instruments Division of NIPER, Chandigarh for spectral and nitrogen analysis respectively.
References
- Rout MK, Mahapatra GN. 2-Naphthyllimino-4-thiazolidone and its derivatives J Am ChemSoc 1955;77 : 2427-31.
- Abbady MA, Awad IMA, Kandeel, MM. Synthesis and antimicrobial activity of 2- arylidencamino-4-phenyloxazoles, azetidinones, thiazolidinones, thiazolines and some spiro-hetrocycles. Indian J Chem 1988;27B : 90-2.
- Fahmy A M, Hassan KM, Khalaf AA, Ahmed RA. Synthesis of some new beta-lactams, 4-thiazolidinones and pyrazolines. Indian J Chem 1987;26B:884-7.
- Joshi N, Patel P, Parekh H. Studies on 4-thiazolidinones. part XX: Synthesis and antimicrobial activity of 2-aryl-5H/methyl/carboxymethy-3-[4-(3,4,5-trimethoxybenzamido)-benzoylyamina] thiazolidin-4-ones. Indian J Chem 1996;35 : 867-70.
- Patolia VN, Patel PK, Baxi AJ. Studies on 4-thiazolidinones. part IX: Synthesis and antimicrobial activity of 2- (2'-Aryl-4-thiazolidinone-3'-ly)-4-(2"-methyl-4"-hydroxy-5"-isopropylphenyl) thiazoles. J Indian ChemSoc 1994;71:683-5.
- Patel PH, Korgaukar SS, Parekh H. Synthesis and antimicrobial activity of 4-thiazolidinones and imidazolinone derivatives bearing 2-amino-thiazole moiety. Indian J HeterocyclChem 1997; 7: 73-6.
- Shahsafi MA, Meshkatalsadat MH, Parekh H. Studies on 4-thiazolidinone:part IV- synthesis and antimicrobial activity of P,P'-bis (5-methyl/carboxymethyl-4-oxo-2-phenylthiazolidin-3-amidomethylamino)-diphenylsulphones. Indian J Chem 1987;26B:803-7.
- Shah VH, Chauhan NA, Parikh AR. Synthesis of 2-Aryl-3-[5'-o-hydroxyphenyl-1',2',4'-thiadiazol-2'-yl]-5-H/methyl-4-thiazolidinones as Antimicrobial Activity. J Indian ChemSoc 1997;74 : 243-4.
- Vashi BS, Metha DS, Shah VH. Comparative studies of methods of evaluating antimicrobial substances. Indian J Chem 1995;34:802-8.
- Mishra P, Gajbhiya A, Jain SK. Synthesis and antibacterial activity of some 4-thiazolidinones Part I. Oriental J Chem 1996;12:325-6.
- Mishra P, Namdeo KP, Jain S, Jain SK. Synthesis and antimicrobial activity of 4-thiazolidinones. Asian J Chem 1999;11 : 55-8.