- *Corresponding Author:
- A. K. Halve
School of Studies in Chemistry, Jiwaji University, Gwalior - 474 011, India
E-mail: halveanand@yahoo.co.in
| Date of Received : | 17 March 2006 |
| Date of Revised : | 24 March 2007 |
| Date of Accepted : | 5 October 2007 |
| Indian J. Pharm. Sci., 2007, 69 (5): 680-682 |
Abstract
Reaction of chloro substituted phenylazo benzenecarbaldehyde(3,4) with primary aromatic amines in ethanol afforded two series of halogenated imines viz; 2-hydroxy-5-(2'chlorophenylazo) benzylidene anilines (5a-d) and 2-hydroxy-5-(4'chlorophenylazo)benzylidene anilines (5e-h) in good yield. The chemical structures were confirmed by IR, 1 H NMR and elemental analysis. The in vitro antibacterial efficacy of synthesized imines (5a-h) was tested against selected screens of Gram-positive and Gram-negative microorganisms. Compound (5g) bearing 4'-chlorophenylazo moiety and 4'-chlorobenzylidineamino functionalities showed broad spectrum in vitro antibacterial activity among the series.
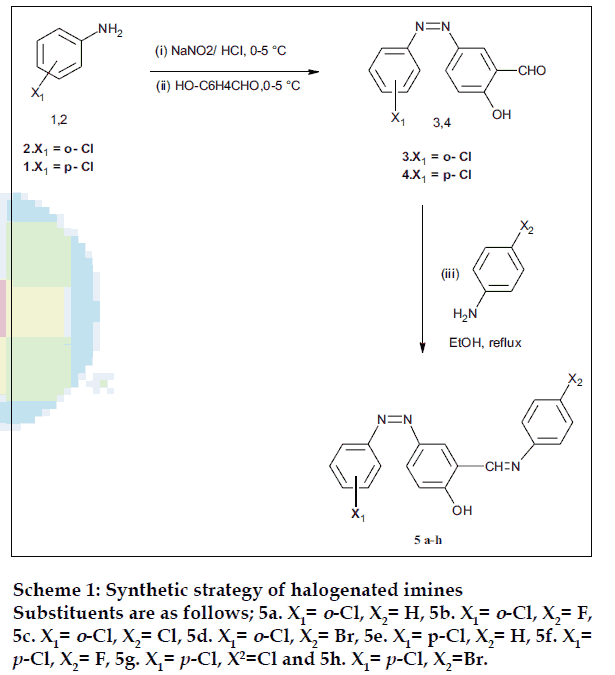
The emergence of antibiotic resistant Gram positive bacteria notably Staphylococci and Enterococci variants, against existing drug necessitates continuing research into new classes of antibacterial [1], organic molecules bearing reactive imine(-CH=N-) pharmacophore provide lead structures [2-4] for further optimization of activity for use in drug development. Furthermore, several derivatives of imines have been shown to be involved in many biological functions and are promising antimicrobial [5-7], antituburcular [8], anticancer [9,10], antiamoebic [11], antiHIV [12] agents and enzyme inhibitors [13,14]. In the present studies, we report the synthesis and antibacterial efficacy of a new series of halogenated imines against a panel of sensitive and resistant Gram positive and Gram- negative pathogens. Two series of halogenated imines, 2-hydroxy-5- (2’chlorophenylazo) benzylidene anilines (5a-d) and 2-hydroxy-5-(4’chlorophenylazo) benzylidene anilines (5e-h) were synthesized via the reaction of 2- hydroxy-5-(2’chlorophenylazo) benzene carbaldehyde (3) and 2- hydroxy-5-(4’chlorophenylazo) benzene carbaldehyde (4) with different primary aromatic amines, respectively (Scheme 1). The constitutions of all the products have been assigned on the basis of elemental analysis, IR and 1H NMR spectral data.
Melting points were determined in open glass capillary tubes and are uncorrected. Thin layer chromatography was used to access the purity of synthesized compounds. IR spectra were recorded on Shimadzu FT-IR Prestige 21 instrument in KBr disc. 1H NMR spectra were recorded on a Varian EM-390 MHz NMR spectrometer in DMSO-d6 using TMS as an internal standard, chemical shift in ppm(δ). Elemental analysis was performed on Carlo Erba 1108 analyzer.
2-Hydroxy-5-(2’chlorophenylazo) benzene carbaldehyde (3) and 2-hydroxy-5-(4’chlorophenylazo) benzene carbaldehyde (4) have been prepared by diazotization and coupling method at 0-5° [15]. 2-Hydroxy-5-(2’- chlorophenylazo) benzylidene aniline (5a) was synthesized by refluxing 0.005 M of compound (3) with an equimolar quantity of aniline in ethanol for 2 h. The reaction mixture was cooled in an ice bath and a drop of sulphuric acid was added. The separated solid was collected, washed and recrystallised from ethanol.
Analytical data of compounds are as follows; 2- hydroxy-5-(2’-chlorophenylazo) benzylidene aniline (5a); mp: 158o; yield: 72%; elemental analysis of C19H14N3OCl: found (calcd, %) C, 59.83 (67.95): H, 3.93 (4.17); N, 11.58 (12.51). IR KBr (ν cm-1): 3583 (O-H), 1622 (C=N), 1592 (N=N); 1H NMR (DMSOd6), δ, ppm: 4.6 (s, 1H, OH), 7.1-7.5 (m,12H, ArH), 9.82 (s,1H, CH=N). 2-Hydroxy-5-(2’-chlorophenylazo)- 4’fluoro benzylidene anilines (5b); mp: 137o; yield: 65%; elemental analysis of C19H13N3OClF: found (calcd, %) C, 6404 (64.49): H, 3.23 (3.67); N, 11.08 (11.88). IR KBr (ν cm-1): 3489 (O-H), 1620 (C=N), 1593 (N=N); 1H NMR (DMSO-d6), δ, ppm: 4.2 (s, 1H, OH), 7.2-7.6 (m,11H, ArH), 9.76 (s,1H, CH=N). 2-Hydroxy-5-(2’chlorophenylazo)-4’-chloro benzylidene anilines (5c); mp: 155o; yield: 74%; elemental analysis of C19H13N3OCl2: found (calcd, %) C, 61.11 (61.62): H, 3.23 (3.51); N, 10.87 (11.35). IR KBr (ν cm-1): 3510 (O-H), 1612 (C=N), 1589 (N=N); 1H NMR (DMSO-d6), δ, ppm: 4.6 (s, 1H, OH), 7.3-7.7 (m,11H, ArH), 9.83 (s,1H, CH=N). 2- Hydroxy-5-(2’chlorophenylazo)-4’bromo benzylidene anilines (5d); mp: 198o; yield: 64%: elemental analysis of C19H13N3OClBr: found (calcd, %) C, 49.77(55.00): H, 2.91 (3.13); N, 10.01 (10.13). IR KBr (ν cm-1): 3573 (O-H), 1618 (C=N), 1590 (N=N); 1H NMR (DMSO-d6), δ, ppm: 4.9 (s, 1H, OH), 7.1-7.5 (m,11H, ArH), 9.54 (s,1H, CH=N).
2-Hydroxy-5-(4’chlorophenylazo) benzylidene anilines (5e); mp: 140o; yield: 75%; elemental analysis of C19H14N3OCl: found (calcd, %) C, 67.13(67.95): H, 4.03 (4.17); N, 12.13 (11.51). IR KBr (ν cm-1): 3559 (O-H), 1613 (C=N), 1605 (N=N); 1H NMR (DMSOd6), δ, ppm: 4.31(s, 1H, OH), 7.4-7.8(m,12H, ArH), 9.17 (s,1H, CH=N). 2-Hydroxy-5-(4’chlorophenylazo)- 4’fluorobenzylidene anilines (5f); mp: 178o; yield: 67%; elemental analysis of C19H13N3OClF: found (calcd, %) C, 64.17(64.49); H, 3.09 (3.67); N, 11.27 (11.88). IR KBr (ν cm-1) 3541 (O-H), 1616 (C=N), 1603 (N=N); 1H NMR (DMSO-d6), δ, ppm: 4.62 (s, 1H, OH), 7.2-7.5 (m,11H, ArH), 9.34 (s,1H, CH=N). 2-Hydroxy-5-(2’chlorophenylazo)-4’chloro benzylidene anilines (5g); mp: 168o; yield: 76%; elemental analysis of C19H13N3OCl2: found (calcd, %) C, 61.43(61.62): H, 3.13 (3.51); N, 10.87 (11.35). IR KBr (ν cm-1) 3512(O-H), 1622 (C=N), 1589 (N=N); 1H NMR (DMSO-d6), δ, ppm: 4.53 (s, 1H, OH), 7.3-7.7 (m,11H, ArH), 9.47 (s,1H, CH=N). 2-Hydroxy- 5-(2’chlorophenylazo)-4’bromobenzylidene anilines (5h); mp: 205o; yield: 63%; elemental analysis of C19H13N3OClBr: found (calcd, %): C, 49.64(55.00) H, 2.97 (3.13); N, 09.86 (10.13). IR KBr (ν cm-1): 3522 (O-H), 1628 (C=N), 1587 (N=N); 1H NMR (DMSOd6), δ, ppm: 4.83 (s, 1H, OH), 7.6-7.9 (m,11H, ArH), 9.54 (s,1H, CH=N).
Antimicrobial activity of imines (5a-h) was tested against a panel of sensitive and resistant Grampositive and Gram- negative strains using disc diffusion assay [16]. Tested microorganisms included Gram-positive (Staphylococcus aureus and Bacillus anthracis) and Gram- negative (Escherichia coli and Klebsiella pneumoniae). For this, sterile filter paper disks (6 mm) impregnated with fixed doses viz; 250 µg/ml of compounds was placed on pre-inocculated surface. The disc bearing plates were incubated within 30 min at 37° for 24 h. After incubation, the inhibition zone diameters were measured. The standard compound chosen was a known, highly active, broad–spectrum antibiotic, chloramphenicol in order to compare the relative activity of synthesized compounds with a standard. The antimicrobial data of tested compounds are presented in Table 1.
| Compound | Gram-positive microorganisms | Gram-negative microorganisms | ||
|---|---|---|---|---|
| S. aureus | B. anthracis | E. coli | K. pneumoniae | |
| 5a | - | 10 | - | 08 |
| 5b | 20 | 25 | - | 10 |
| 5c | 20 | 24 | 10 | 08 |
| 5d | - | 08 | - | - |
| 5e | 25 | 18 | 08 | 11 |
| 5f | 28 | 24 | - | 08 |
| 5g | 33 | 28 | 11 | 18 |
| 5h | 10 | 08 | - | 08 |
| Chloramphenicol | 35 | 30 | 16 | 25 |
*Diameter of zone of inhibition in mm. Control (DMF) = No activity. Both, test compound and standard were tested at 250 µg/ml
Table 1: Results of in vitro antibacterial activity* of newly synthesised imines (5a-h).
The result of antibacterial screening reveals that compounds (5e-g) with para chloro phenyl azo moiety exhibited potent Gram-positive activity as compared to ortho chloro derivatives (5a, 5b) and (5c). Among 4’-chloro derivatives compound (5g) with X1, X2= para chloro substituents distinguished itself for its impressive potency against Gram-postive species viz; Staphylococcus aureus and Bacillus anthracis. Taking Gram- negative data, (5g) showed highest activity against Klebsiella pneumoniae and significant inhibition against Escherichia coli. Moreover, (5f) with 4’-fluoro benzylidene amino group exhibited promising results against Gram-positive bacteria while its Gramnegative activity was considerably lower. In addition, compound (5e) showed remarkable anti staphylococcal activity with a marked decrease in Gram- negative values. However, 4’-bromo substituents in (5d) and (5h) resulted in complete reduction in antibacterial activity of imine derivatives. On the other hand, among ortho chloro derivatives (5a-d), a compound (5b) was the only one to show significant Gramnegative efficacy, while all other derivatives were completely inactive.
Thus, it has been concluded that synthesized compounds showed pronounced antibacterial activity against tested bacterial strains due to stable imine linkage and their efficacy increases by chloro functionality at para position of benzene and phenylazo nuclei whereas unsubstituted benzylidene amino group, ortho chloro and para bromo substitutents subsides the bacterial effectiveness of imine compounds. Hence compound (5g) seemed to be an attractive candidate for further development as it is the most active compound among the series having broad spectrum antibacterial activity against the tested organisms. Further extension of this research work will be directed to determine the molecular level target for this compound in the microorganism.
Acknowledgements
Sincere thanks are due to the Dean, Birla institute of Medical Research and College of Life Sciences, Gwalior, for providing facilities and to the UGC, New Delhi, for financial assistance.
References
- Butler, M.M., Lamarr, W.A., Skow, D.J., Matorina, I., Lamothe, S. and Storer, R., J. Med. Chem ., 2005, 48, 7063.
- Dax, C., Coincon, M., Syguseh, J. and Blonski, C., Biochem ., 2005, 44, 7063
- Sugiyama, L., Kittaka, A., Takayama, H., Miisugu, T., Ida, Y and Kurodo, R., Bioorg. Med. Chem. Lett. 2003, 13, 2847.
- Vaghasiya, Y.K. Nair, R., Soni, M., Baluja, S. and Chanda, S., J. Serb. Chem. Soc ., 2004, 69, 991.
- Paneerselvan, P., Nair, R.R., Gudaparthi, V., Subramanian, E.H. and Sridhar, S.K., Eur. J. Med. Chem. , 2005, 40, 225.
- Ramesh, R. and Maheshwaran, S., J. Inorg. Biochem., 2004, 17, 115.
- Chohan, Z.H. and Farooq, M., J. Enz. Inhib. Med. Chem., 2002, 17, 1.
- Hearn, M.J. and Cynamon, M.H., J. Antimicrob. Chemother., 2004, 53, 185.
- Ferruti, P., Bianchi, S., Ranucci, E., Chiellini, F. and Caruso, V., Macromol. Biosci., 2005, 5. 613.
- Marin, J.J., Macias, R.I., Briz, O., Perez, M.J. and Serrano, M.A., Ann. Hepatol., 2005, 4. 70.
- Bharti, N., Maurya, M.R., Naqvi, F. and Azam, A., Bioorg, Med. Chem. Lett. 2001, 11, 1099.
- Sridhar, S.K., Pandeya, S.N. and De Clerque, E., Bull. Chem., Farm. , 2001, 140, 302.
- Puccetti, L., Fazolis, G., Vullo, D., Chohan, Z.H., Scozzafava, A. and Supuran, C.T., Bioorg. Med. Chem. Lett., 2005, 15, 3096.
- Supuran, C.T. and Clare, B.W., Eur. J. Med. Chem ., 1998, 33, 489.
- Halve, A.K., Gour, P., Dubey, R., Bhadauria, D. and Bhaskar, B., Indian J. Chem., 2005, 44B, 2163.
- Bauer, A.W., Kirby, W.M.M., Sherris, J.C. and Turck, M., Ann. J. Path., 1966, 36, 493.