- Corresponding Author:
- Shobha R. Desai
Department of Chemistry, SS Arts College and TP Science Institute, Sankeshwar - 591 313
E-mail: dr.shobha.desai@gmail.com
| Date of Submission | 5 June 2010 |
| Date of Revision | 8 December 2010 |
| Date of Acceptance | 6 February 2011 |
| Indian J Pharm Sci, 2011, 73 (1): 115-120 | |
Abstract
In this study, various 3-β-[(N-benzenesulphonyl/tosyl)-4-(un) substituted anilino]ethyl-4-amino-5-mercapto-4(H)- 1,2,4-triazoles (5a–f), with biologically active 'sulphonamide' moiety as the side chain have been prepared. The structures of the newly synthesised compounds have been established on the basis of their spectral data and elemental analysis. All the compounds were evaluated for antimicrobial activities against Escherichia coli, Bacillus cirroflagellosus, Aspergillus niger and Colletotrichum capsici. Most of the compounds investigated exhibited significant antifungal activity against Colletotrichum capsici, even greater than fluconazole, the standard used. Only two compounds 3f (59%) and 5e (67%), have shown moderate antituberculosis activity. All the triazoles exhibited moderate degree of antiinflammatory activity and least ulcerogenecity. Most of the compounds have shown significant analgesic activity (81.02–120.72%) in comparison with aspirin (49.39%). In the MES method, only compound 3e exhibited a protection of 66.66%, whereas others exhibited minimum protection of (33.33%).
Keywords
1,2,4--Triazoles, analgesic, antiinflamatory, antimicrobial, anticonvulsant activities
Various 4-amino-5-mercapto-1,2,4-triazoles and their derivatives are associated with diverse pharmacological activities such as antibacterial [1,2], antifungal [1-3], antituberculosis [3,4], antiinflammatory [5,6], analgesic [2-7], anticonvulsant [8], antiviral [4-9] and antitumor activities [4]. Sulfonamides were the first effective chemotherapeutic agent to be employed systematically for the prevention and cure of bacterial infection in human as broad spectrum antimicrobials. Sulphonamides have been also reported as, antidiabetic [10], antipyretic [11] and plant growth retarding agent [12].
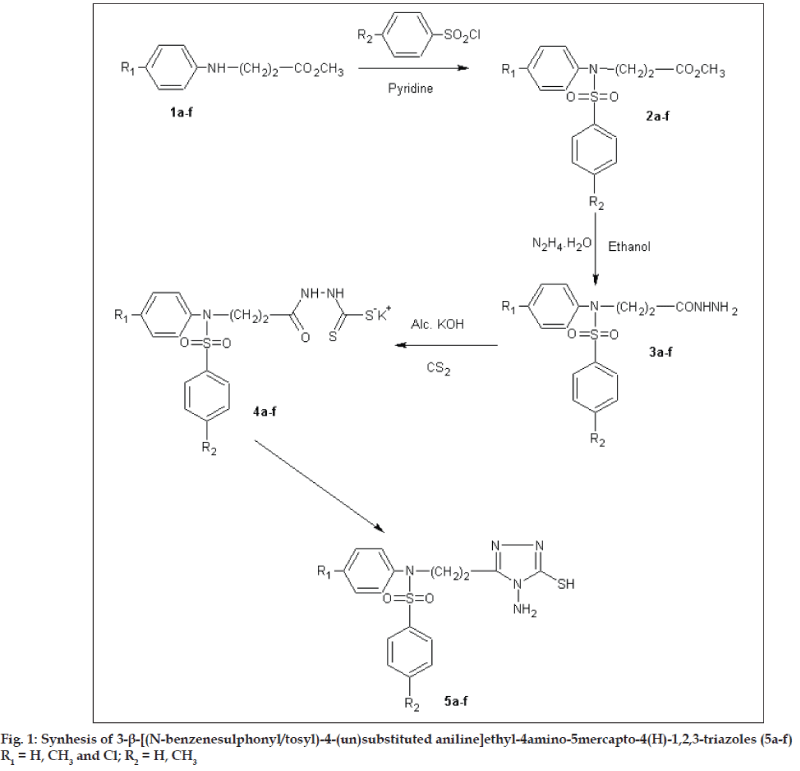
Encouraged by these observation and by the established pharmacological activities of 1,2,4-triazoles, it was contemplated to incorporate the biologically active 'sulfonamide moiety' at the 3rd position of 1,2,4-triazole ring, with a hope of obtaining triazoles with improved pharmacological activities. To enable further evaluation of the potential usefulness of 1,2,4-triazoles and in continuation of our search for 1,2,4-triazoles as pharmacologically active heterocycles [5,6], we report here in the synthesis of some new 3-b- [(Nbenzenesulphonyl/ tosyl)-4-(un) substituted anilino] ethyl-4-amino-5-mercapto-4(H)-1,2,4-triazoles (5a-f) with a view to achieve better antiinflammatory activity and least ulcerogenecity. All the compounds were evaluated for antimicrobial activities against Escherichia coli, Bacillus cirroflagellosus, Aspergillus niger and Colletotrichum capsici. The same compounds were evaluated for antituberculosis activity against Mycobacterium tuberculsosis H37Rv strain. The parent triazoles were evaluated for pharmacological activities such as antiinflammatory, analgesic, ulcerogenecity and anticonvulsant activities. The synthetic route for the title compounds is depicted as fig. 1.
In the present investigation, methyl β- [(Nbenzenesulphonyl/ tosyl)-4-(un)substituted anilino] propionates (2a-f) were prepared according to literature methods [6,13]. Esters (2a-f), when refluxed with hydrazine hydrate yielded the corresponding hydrazides (3a-f). Potassium β- [(N-benzenesulphonyl/ tosyl)-4-(un)substituted anilino] propionyl dithiocarbazinates (4a-f), were obtained by stirring hydrazides (3a-f) with alcoholic potassium hydroxide and carbon disulphide. Potassium salts (4a-f), on reaction with two fold excess of hydrazine hydrate yielded 3-β- [(N-benzenesulphonyl/tosyl)-4-(un) substituted anilino]ethyl-4-amino-5-mercapto-4(H)- 1,2,4-triazoles (5a-f). The structures of the newly synthesised compounds were confirmed by elemental and spectral (IR, 1H-NMR and mass) analysis. The physicochemical properties are enumerated in Table 1.
| Compd no. | R1 | R2 | MP(°) | Yield(%) | Molecular* formula |
|---|---|---|---|---|---|
| 3a | H | H | 103-04 | 70 | C15H17N3O3S |
| 3b | H | CH3 | 140-42 | 75 | C16H19N3O3S |
| 3c | CH3 | H | 89-90 | 60 | C16H19N3O3S |
| 3d | CH3 | CH3 | 117-18 | 64 | C17H21N3O3S |
| 3e | Cl | H | 65-67 | 50 | C15H16N3O3SCl |
| 3f | Cl | CH3 | 140-40 | 52 | C16H18N3O3SCl |
| 5a | H | H | 187-88 | 44 | C16H17N5O2S2 |
| 5b | H | CH3 | 210-12 | 45 | C17H19N5O2S2 |
| 5c | CH3 | H | 152-54 | 40 | C17H19N5O2S2 |
| 5d | CH3 | CH3 | 165-67 | 41 | C18H21N5O2S2 |
| 5e | Cl | H | 112-14 | 30 | C16H16N5O2S2Cl |
| 5f | Cl | CH3 | 107-09 | 35 | C17H18N5O3S2Cl |
*All the compounds were analyzed for C, H and N. The experimental values were within ±0.04% of the calculated value.
Table 1: Physicochemical properties of hydrazides 3a-f and 1,2,4-Triazoles 5a-f
Melting points were determined in open capillaries and are uncorrected. IR spectra in KBr, were recorded on a Perkin Elmer spectrophotometer and 1H-NMR spectra on a Varian 300 MHz NMR spectrometer using TMS as an internal standard (chemical shifts in δ ppm). Mass spectra were recorded on Finnigan MAT 8230 mass spectrometer. The starting materials, methyl β- [N-benzenesulphonyl/ tosyl)-4-(un) substituted anilino] propionates (2a-f) were prepared according to literature methods [6,13].
Sulphonylation of propionates (2a-f), was carried out by adding benzenesulphonylchloride (8.8 g, 0.05 mol) drop wise with cooling to a stirred solution of methyl-β-anilino propionate (0.05 mol) in dry benzene (10 ml) and pyridine (5.9 g, 0.075 mol). Reaction mixture was stirred for 48 h (fig. 1). The reaction mixture was diluted with water and extracted with ether. The ethereal layer was washed with 10% hydrochloric acid, followed by 5% aqueous potassium hydroxide, water and a saturated solution of sodium chloride. The ether layer was then dried over anhydrous sodium sulphate and the solvent was removed under reduced pressure to get the sulphonylated ester (2a-f).
β- [(N-benzenesulphonyl/tosyl)-4-(un)substituted anilino] propionic acid hydrazides (3a-f), were prepared by refluxing a mixture of methyl, β- [(Nbenzene sulphonyl)anilino]propionates(2a-f), (0.05 mol), hydrazine hydrate 99% (2.25 g, 0.05 mol) in ethyl alcohol (50 ml) for 6-8 h. Concentration in vacuum left a residue, which was poured onto crushed ice. The solid separated was filtered, washed with water, dried and crystallised from ethyl alcohol (3a-f). Physicochemical properties of same compounds are enumerated in Table 1. 3d IR (KBr) υ cm-1: 3500 (s), 3340 (m) (NH2), 3110 (b) (NH), 2990 (m) (CH2), 1730 (s), 1560 (m) (>C=O), 1390 (s) (SO2); 3e: IR (KBr) υ cm-1: 3580 (s), 3320 (m) (NH2), 3060 (b) (NH), 2930 (m) (CH2), 1640 (s), 1600 (m) (>C=O), 1400 (m) (SO2). 1HNMR: (DMSO-d6): 3b: δ 2.41 (t, 2H, -CH2-), 2.50 (s, 3H, CH3), 3.86 (t, 2H, -N-CH2-), 4.6 (s, 2H, NH2), 7.00- 7.62 (m, 9H, ArH), 8.4 (s, 1H, NH). 3d: d 2.33 (t, 2H, -CH2-), 2.36 (s, 3H, CH3), 2.42 (s, 3H, CH3), 3.80 (t, 2H, -N-CH2-), 4.5 (s, 2H, NH2), 6.88-7.48 (m, 8H, ArH), 8.5 (s, 1H, NH).
General procedure for preparation of potassium β- [(N-benzenesulphonyl/tosyl)-4-(un)substituted anilino]propionyl dithiocarbazinates (4a-f), involved the addition of carbon disulphide (2.28 g, 0.03 mol) drop wise to a clear solution of potassium hydroxide (1.68 g, 0.03 mol) in absolute ethanol (15 ml) and β- [(N-benzenesulphonyl/tosyl)-4-(un) substituted anilino]propionic acid hydrazide (3a-f) (0.02 mol) with stirring and cooling in ice. The reaction mixture was stirred continuously for 12 h and diluted with anhydrous ether (200 ml). The potassium dithiocarbazinate that separated was filtered, washed several times with anhydrous ether and dried in vacuum. The potassium salt(s) (4a-f) obtained in quantitative yield, were moisture sensitive and hence used directly for the preparation of the corresponding triazoles without further purification.
3 - β - [ (N-benzene sulphonyl/tosyl) -4- (un) substitute danilino]ethyl-4-amino-5-mercapto-4(H)- 1,2,4,-triazoles (5a-f), were prepared by refluxing a clear solution of potassium salts of β- [(N benzene sulphonyl/ tosyl)-4-(un)substituted anilino) propionyl dithiocarbazinates (4a-f), (0.02 mol) in water (10 ml) and hydrazine hydrate 99% (2.0 g, 0.04 mol) for 5-6 h. The color of the mixture turned to green with the evolution of hydrogen sulphide gas (lead acetate paper test and odor). The clear solution was treated with decolorising charcoal, filtered, cooled in ice and then carefully acidified with acetic acid. The precipitated solid was filtered, washed with water, dried and crystallised from ethanol. 5b, IR (KBr) υ cm-1: 3500 (s), 3210 (m) (NH2), 3000 (b) (NH), 2850 (s) (CH2), 1650 (s), 1540 (m (>C=C<, >C=N-) 1430 (m) (>C-N), 1190 (s), 1120 (m), (>C=S), 1390 (s) (SO2); 5b: 1HNMR (DMSO-d6): δ 2.05 (t, 2H, -CH2), 2.42 (s, 3H, CH3), 3.99 (t, 2H, -N-CH2-), 4.61 (s, 1H, NH2), 10.59 (br.s, 1H, NHC= S), 7.00-7.48 (m, 8H, ArH). 5b: MS: (m/z, Rel. Abund): 389 (M+, 10), 260 (56), 248 (12), 155 (82), 106 (22), 91 (100), 78 (18). 5c: 1HNMR: (DMSO-d6): δ 2.34 (s, 3H, CH3), 2.94 (t, 2H, -CH2-), 3.98 (t, 2H, -N-CH2-), 4.62 (s, 2H, NH2), 10.81 (br.s, 1H, NH-C=S), 6.89-7.61 (m, 8H, ArH). 5d: MS: (m/z, Rel. Abund): 404 (15), 285 (60), 250 (20), 160 (90), 125 (20), 106 (100), 91 (90), 78 (20). 5e: IR (KBr) υ cm-1: 3510 (s), 3210 (m) (NH2), 3010 (b) (NH), 2910 (m) (CH2), 1670 (s), 1620 (m) (>C=C<, >C=N-) 1490 (m) (>C-N), 1230 (s), 1100 (m), (>C=S), 1430 (s) (SO2). Physicochemical properties are enumerated in Table 1.
After establishing the physicochemical properties of triazoles (5a-f), the compounds were subjected to pharmacological screening. Newly synthesised triazoles 5a-f, were first subjected for acute toxicity study in order to determine their, LD50. The study was carried out according to the method of Miller and Tainter [14]. The graphically calculated LD50 values of the newly synthesised compounds were found to be 2238±34.3 (5a-d), 1849±23.1 (5e-f).
The antiinflamatory activity of triazoles 5a-f, was studied by carrageenan-induced rat paw edema method due to Winter et al [15], cotton pellet induced granulation tissue formation method due to Meir, et al. [16] and adrenal weight change method. To determine the severity of the ulcers, an arbitrary scoring system [17] was followed. Analgesic activity of triazoles 5a-f, was carried out by radiant heat induced rat tail flick method [18]. Anticonvulsant activity was studied by MES method [19,20]. The percentage protections were then assessed by Fischer's exact test [21].
All the compounds were evaluated for antimicrobial activities against Gram negative bacterium Escherichia coli, Gram positive bacterium Bacillus cirroflagellosus, and fungi Aspergillus niger and Colletotrichum capsici by cup plate method [22]. Antimicrobial activity is calculated as relative percent inhibition (with reference to the standard). DMF was used as solvent control. Cotrimoxazole (Trimethoprim 500 mg and Sulphamethoxazole 800 mg) and Diflucan (Flucanazole) are used as standards for antibacterial and antifungal respectively. Antimicrobial activity is expressed as relative percent inhibition in comparison with the standard.
Some of the newly synthesised compounds were tested for their antituberculosis activity against M. tuberculosis H37Rv by Bactec 460 radiometric system at Southern Research Institute, Frederick Research Center, Frederick MD. All the compounds were tested at a concentration of 12.5 μg/ml. Rifampicin was used as standard (97%, at 12.5 μg/ml).
In the carrageenan-induced rat paw edema method all the compounds exhibited moderate degree of antiinflammatory activity in comparison to the aspirin, 5b (75.80%, 65.60%, 57.60%), 5c (-, 69.63%, 67.60) and 5e (43.56, 51.40%, 67.58%), in comparison with the aspirin (54.84%, 55.05%, 73.81%) after 1, 3 and 6 h respectively. However remaining compounds 5d and 5f exhibited minimum antiinflammatory activity (19–32.66%), (Table 2). The same triazoles, however exhibited antiinflammatory activity (53.81-43.54) in the cotton pellet method better than the aspirin (41.88), except compound 5f (33.16). This has been further substantiated by the fact that the suppression of the adrenal weights of majority of the compounds are comparable (51.53- 45.37) with that of the standard, aspirin (50.13%), except compound 5b (13.84), Table 2. During the present investigation all the triazoles except 5c (ulcer index, 40.00), have shown lesser degree (10–23.33) of ulcerogenecity, as compared to the aspirin (43.33), Table 2.
| Comp no. | Dose(mg/Kg,b.w.) | Ulcerindex | Antiinflammatory activitya Percentage Inhibition (p) | Analgesic activitya percent protection (p) | Anti convulsantb activity | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Carrageenan method | Cotton pellet method | Adrenal weight method | Rat tail flick method | MES protection | ||||||
| 1 h | 3 h | 5 h | ||||||||
| Std(a/b) | 200 | 43.33 | 54.84 | 55.05 | 73.81 | 41.89 | 50.13 | 49.39 | 100 | |
| (<0.02) | (<0.01) | (<0.001) | (<0.001) | (<0.01) | (<0.001) | (<0.01) | (>1, s) | |||
| 5a | 200 | 10 | --------- | 4.85 | 34.66 | 53.81 | 51.53 | 113.32 | 0 | |
| (ns) | (>0.5) | (>0.1) | (<0.001) | (<0.001) | (<0.001) | (ns) | ||||
| 5b | 200 | 23.33 | 75.80 | 65.60 | 57.60 | 43.55 | 13.84 | 105.24 | 0 | |
| (>0.5) | (<0.05) | (<0.001) | (<0.01) | (<0.001) | (>0.5) | (<0.001) | (ns) | |||
| 5c | 200 | 40 | --------- | 69.63 | 67.60 | 52.60 | 51.00 | 81.02 | 33 | |
| (<0.02) | (<0.02) | (<0.001) | (<0.001) | (<0.001) | (<0.001) | (<0.03, ns) | ||||
| 5d | 200 | 20 | 11.42 | 19.02 | 30.18 | 52.81 | 23.04 | 120.72 | 0 | |
| (>0.5) | (>0.5) | (>0.1) | (<0.05) | (<0.001) | (<0.1) | (<0.001) | (ns) | |||
| 5e | 150 | 10 | 43.56 | 51.40 | 67.58 | 50.40 | 49.82 | 96.5 | 66 | |
| (ns) | (<0.1) | (<0.001) | (<0.001) | (<0.001) | (<0.001) | (<0.001) | (>0.22, s) | |||
| 5f | 150 | 13.33 | 19.36 | 19.02 | 32.66 | 33.16 | 45.37 | 93.81 | 33 | |
| (>0.5) | (>0.5) | (>0.1) | (>0.1) | (<0.01) | (<0.001) | (<0.001) | (<0.03, ns) | |||
Std (standards) a - aspirin and b- phenytoin sodium. LD50±D (Miller and Tainter method): 2238±34.30 (for 5a, 5b,5c, 5d) and 1849±23.10 (for 5e, 5f); a- standard used for antiinflammatory and analgesic activities; b- standard used for anticonvulsant activity; statistical differences between the treatment and the control group of animals were evaluated by Student's t test for evaluation of the analgesic and antiinflammatory activity; P <0.05, s- significant, ns- non significant.
Table 2: Pharmacological activities of 3-β- [(N-Benzenesulphonyl/ Tosyl)-4-(UN)Substituted Anilino] Ethyl-4-Amino-5-Mercapto-4(H)-1,2,4,-Triazoles (5A-F)
In the radiant heat-induced rat tail flick method, all the compounds have exhibited most significant analgesic activity (P<0.001) in comparison with the aspirin (49.39, P<0.01) and the activity given in their decreasing order is 5d (120.72%), 5a (113.32), 5b (105.24), 5e (96.5), 5f (93.81) and 5c (81.02), (Table 2). In the maximum electroshock seizure MES method, only compound 5e has shown a moderate protection of 66% in comparison with the standard sodium phenytoin (100%). Compounds 5c and 5f exhibited 33% protection. Rest of the compounds failed to protect from MES, (Table 2).
Amongst all the compounds tested (3a-f) and (5a-f), majority have exhibited greater antifungal activity against Colletotrichum capsici. Antifungal activity, expressed as relative percent inhibition given in parentheses is enumerated in the decreasing order is 3f, 5a (544.44), 3c (439.39), 3d (302.00), 3e, 5f (206.06), 5b, 5c (126.94), 5d (64.65) and 3b (19.19), (Table 3). However the same compounds exhibited minimum antifungal activity (14–29%) against Aspergillus niger, except compounds 5a (158.56), 5c (78.76) and 5d (72.20) (Table 3). All the compounds exhibited minimum antibacterial activity (14–57.8%) against Bacillus cirroflagellosus. Some of the compounds 3e (100), 5c (73.67), 5b, 5d (51.11), 3a, 3b (41.25) have shown moderate antibacterial activity against Escherichia coli. Rests have shown minimum activity (Table 3). Only two compounds 5e (67) and 3f (59) exhibited moderate antituberclosis activity in comparison to the standard Rifampicin which has 97% at 12.5 μg/ml. All the remaining compounds exhibited minimum antituberculosis activity (16-48%) against, M. tuberculosis H37Rv, (Table 3).
| Comp no. | R1 | R2 | Antimicrobial activity (Relative percent inhibition) | Antituberculosis activity* M. tuberculosis (Percent inhibition) | |||
|---|---|---|---|---|---|---|---|
| E. coli | B. cirro | A. niger | C. capsici | ||||
| 3a | H | H | 41.25 | ------ | 59.84 | 156.57 | 33 |
| 3b | H | CH3 | 41.25 | 36.99 | ------ | 19.19 | 22 |
| 3c | CH3 | H | 67.69 | 29.85 | 28.96 | 439.39 | 28 |
| 3d | CH3 | CH3 | 51.12 | 8.08 | ------ | 302.00 | 41 |
| 3e | Cl | H | 100.00 | 14.97 | 7.34 | 206.00 | 16 |
| 3f | Cl | CH3 | 11.17 | 29.85 | ------ | 544.44 | 59 |
| 5a | H | H | ------ | ------ | 158.56 | 544.44 | 20 |
| 5b | H | CH3 | 51.11 | 14.97 | 24.71 | 126.94 | 22 |
| 5c | CH3 | H | 73.67 | ------ | 78.76 | 126.94 | 26 |
| 5d | CH3 | CH3 | 51.11 | ------ | 72.2 | 64.65 | 48 |
| 5e | Cl | H | 20.68 | 57.8 | 13.51 | 126.93 | 67 |
| 5f | Cl | CH3 | 11.16 | 44.81 | ------ | 206.06 | __ |
*Rifampicin as standard (97%, at 12.5 μg/ml), Concentration (antitubercular activity) - MIC: 12.5 μg/ml (Bactec 460 radiometric system). Concentration (antimicrobial activity)- 100 μg/ml (Cup plate method). DMF as solvent control; Cotrimoxazole (trimethoprim 500 mg and sulphamethoxazole 800 mg) and flucanazole as standards for antibacterial and antifungal, respectively. Relative percent inhibition = [100×(X-Y)/(Z-Y)]; where X , Y and Z are total area of inhibitions in test, solvent (DMF) and standard respectively; Area= πr2 ; where r= radius of zone of inhibition.
Table 3: Antimicrobial and antitubercular activity of hydrazides 3a-f and 1,2,4-Triazoles 5a-f
Acknowledgements
Authors thank the Chairman, Department of Chemistry, Karnataka University, Dharwad, for providing the necessary facilities. Authors also thank the Heads of RSIC-CDRI, Lucknow, TIFR-Bombay, RSIC-IIT, Bombay for spectral analysis and the authorities of TAACF, Southern Research Institute, Birmingham for screening of the compounds for antituberculosis activity. Authors UVL and SRD are thankful to CSIR and UGC, New Delhi respectively for the financial assistance in the form of SRF and FIP.
References
- Banday MR, Rauf A. Substituted 1,2,4-triazoles and thiazolidinones from fatty acids: Spectral characterization and antimicrobial activity. Indian J Chem 2009;48B:97-102.
- Ravindra KC, Vagdevi HM, Vaidya VP. Synthesis, characterization and pharmacological studies on some triazolothiadiazines and triazolothiadiazoles containing naphtho[2,1-b]furan. Indian J Chem 2008;47B:1271-6.
- Dave TK, Purohit DH, Akbari JD, Joshi HS. Synthesis and pharmacological study of thiazolidinones and mannich bases of 4-amino-3-mercapto-5-pyridin-3'-yl-[1,2,4]triazole. Indian J Chem 2007;46B:352-6.
- Kumar PV, Rao VR. Synthesis and antitubercular, antiviral and anticancer activity of 3-(3-mercaptoalkyl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-6-yl)chromen-2-one and its derivatives. Indian J Chem 2008;47B:106-11.
- Hosur MC, Talawar MB, Bennur SC, Patil PA. Synthesis and antiinflammatory activity of 3-substituted-4-amino-5-mercapto-4(h)-1,2,4-triazole. Indian J Pharm Sci 1993;55:86-90.
- Talawar MB, Bennur SC, Kankanwadi SK, Patil PA. Antiinflammatory activity of some new 3-substituted-4-amino-5-mercapto-4(h)-1,2,4-triazoles. Indian J Pharm Sci 1995;57:194-7.
- Tozkoparan B, Aytaç SP, Aktay G. Novel 3,6-Disubstituted 7H-1,2,4-Triazolo[3,4-b][1,3,4]thiadiazines: Synthesis, Characterization, and Evaluation of Analgesic/Antiinflammatory, Antioxidant Activities. Arch Pharm (Weinheim) 2008 ; 342:291-8.
- Kane JM, Staeger MA, Dalton CR, Miller FP, Dudley MW, Ogden AM, et al. 5-Aryl-3-(alkylthio)-4H-1,2,4-triazoles as selective antagonists of strychnine induced convulsions and potential antispastic agents. J Med Chem 1994:37:125-32.
- Abdel-Aal MT, El-Sayed WA, El-Kosy SM, El-Ashry el SH. Synthesis and antiviral evaluation of novel 5-(N-Aryl-aminomethyl-1,3,4-oxadiazol-2-yl)hydrazines and their sugars, 1,2,4-triazoles, tetrazoles and pyrazolyl derivatives. Arch Pharm (Weinheim) 2008 ; 341:307-13.
- Xiang J, Wan ZK, Li HQ, Ipek M, Binnun E, Nunez J, et al. Piperazine Sulfonamides as Potent, Selective, and Orally Available 11â-Hydroxysteroid Dehydrogenase Type 1 Inhibitors with Efficacy in the Rat Cortisone-Induced Hyperinsulinemia Model. J Med Chem 2008;51:4068-71.
- Penning TD, Talley JJ, Bertenshaw SR, Carter JS, Collins PW, Docter S, et al. Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide (SC-58635, celecoxib). J Med Chem 1997;40:1347-65.
- Bhattacharya G, Herman J, Delfín D, Salem MM, Barszcz T, Mollet M, et al. Synthesis and Antitubulin Activity of N1- and N4-Substituted 3,5-Dinitro Sulfanilamides against African trypanosomes and Leishmania. J Med Chem 2004;47:1823-32.
- Sangwan NK, Kelkar PM, Rastogi SN, Anand N. Antifertility agents: Part XLIII-Synthesis of 3-aryl-4,5-dihydro-2-substituted-5-tosyl-2H-pyrazolo(4,3-c)quinolines and 2,4-dihydro-3-phenyl(1) benzopyrano(4,3-c)pyrazoles and their derivatives. Indian J Chem 1985;24B:639.
- Miller LC, Tainter ML. Graphical method for determination of LD 50 .ProcSocExptBiol Med 1944;57:261-71.
- Winter CA, Risley EA, Nuss GW. Carrageenan-induced edema in hind paw of the rats as an assay for antiiflammatory drugs. ProcSocExptBiol Med 1962;111:544-7.
- Meir R, Schuler W, Desaulles P. On the mechanism of cortisone inhibition of connective tissue proliferation.Experientia 1950;6:469-71.
- Gupta MB, Nath R, Gupta GP, Bhargava KP. A study of the antiulcer activity of diazepam and other tranquilosedatives in albino rats.ClinExpPharmacol Physiol 1985;12:61-6.
- Davies OL, Reventos J, Welpole AL. A method for the evaluation of analgesic activity using rats. Br J PharmacolChemother 1946;1:255-64.
- Holland GF, Jaeger DA, Wagner RL, Laubach GD, McLamore WM, P'anSy. Hypoglycemic activity in a series of 1-aryl-3-arylsulphonylureas. J Med Pharm Chem 1961;3:99-110.
- Kadlimatti SH, Joseph T. Influence of lithium on the anticonvulsant activity of carbamazepine. Indian J Physiol Pharmacol 1987; 31:35-41.
- Armitage P. Statistical Methods in Medical Research. Oxford, UK: Blackwell Scientific Publications; 1971. p.135-8.
- Seeley HW, Denmark PV. Microbes in action editors. A Lab Manual of Microbiology, 2nd ed. New York: W. H. Freeman And Co; 1981. p. 106-07.