- *Corresponding Author:
- R. R. Kamble
Department of Studies in Chemistry, Yuvaraja’s College, University of Mysore, Mysore-570 005. India
E-mail: ravichem1234@rediffmail.com
| Date of Submission | 4 February 2005 |
| Date of Revision | 3 June 2005 |
| Date of Acceptance | 18 March 2006 |
| Indian J Pharm Sci, 2006, 68 (2): 249-253 |
Abstract
The title compounds were synthesised by using cyclization of a, b-unsaturated ketones and thiourea, in presence of a base. All the newly synthesised compounds have been characterised by elemental analyses, spectral data, and screened for antibacterial, antifungal, anti-inflammatory, and anticonvulsant activities.
The synthesis and biological properties of 3-arylsydnones incorporated with wide variety of potent molecules, have been reported from our laboratory, earlier [1-5]. During the present work, we have utilised sydnones as synthons for heterocyclic synthesis, as they readily undergo ring transformation to various heterocycles, by 1,3-dipolar cycloadditions [6]. One such reaction has been reported [7], wherein 3-arylsydnones have been converted to the corresponding 1,3,4-oxadiazoles, by a simple one-pot reaction (85% yield). The ease of formation of these compounds from sydnones, increases the synthetic utility of this ring conversion. Only few such oxadiazoles were obtained with difficulty and in very low yield, starting from phenylhydrazines [8]. In view of the fact that sydnone ring serves as a versatile synthon for such compounds, which are difficultly obtained by other methods, we thought of preparing some other substituted 1, 3, 4oxadiazoles. The present work was extended to pacetylphenylsydnone 1, and it’s α, β-unsaturted ketones, which were used to build up biheterocycles containing biologically active heterocycles viz., pyrimidine and oxadiazole. Pyrimidine derivatives possess wide range of pharamacological activities [9,10]. Pyrimidine, an important ring system found in the nucleic acid, antibiotics, antimalarials, anticancer, anti-HIV, and anti-inflammatory drugs [11] is associated with diverse biodynamic properties, and is of interest with context of drug development, because of its structure similarity to purines. In the recent past, it has been observed and reported, that considerable antibacterial and antifungal activity has been exhibited by 1,3,4-oxadiazole derivatives, suitably substituted at 2 and 5 positions [12-15]. Because of biological activities, these heterocycles are of great interest to medicinal chemists for molecular manipulation, and to biologists for further pharmacological evaluation.
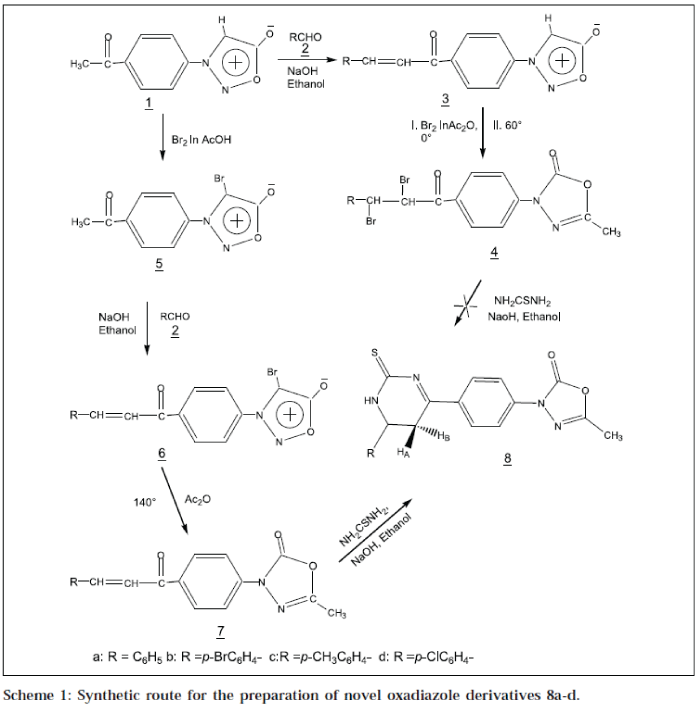
In view of these observations, we herein report the synthesis and pharmacological screening of 5-methyl-3[p-(6'-aryl-2'-thioxo-1',2',5',6'-tetrahydro-pyrimidin-4'-yl)phenyl]-3H-2-oxo-Δ4-1,3,4-oxadiazoles 8a-d. Synthesis of biheterocycles, which is effected sequentially, requires bifunctional precursors, which are not always readily accessible. The compound 1, which can be conveniently prepared from P-amino acetophenone, serves as an important synthetic precursor for the synthesis of title compounds.
As shown in Scheme 1, our initial synthetic strategy involved the preparation of key intermediate Pacetylphenylsydnone1 [16], which on condensation with an aromatic aldehyde 2a-d in ethanol, afforded 3-[P-(3'-arylacryl-1'-oyl)-phenylsydnone 3a-d. Compound 3a-d, on bromination in acetic anhydride, sydnone ring underwent 1,3-dipolar cycloaddition to give 3-[P-(2',3'-dibromo-3'-arylpropion-1'-yl)-phenyl]-5-methyl-3H-2-oxo-Δ4-1,3,4oxadiazole 4a-d. During cycloaddition reaction, the chalcone moiety was brominated, due to excess of bromine. Dibromo chalcone 4a-d, on treatment with thiourea, did not form the target compound 8a-d. Therefore, this method of ring transformation of sydnone into oxadiazoline and approach for synthesis of 8a-d, was not advantageous due to the bromination of chalcone moiety.
The strategy of synthesis was changed. The starting material 1, was brominated in presence of acetic acid, at room temperature to 4-bromo-3- (P-acetyl)-phenylsydnone 5, which upon treatment with aromatic aldehydes 2a-d in ethanol, afforded 4-bromo-3-[P-(3'-aryl-acryl-1'-oyl)] phenylsydnone 6a-d. Compound 6a-d, upon heating in presence of acetic anhydride at 135° sydnone ring, underwent 1,3-dipolar cycloaddition along with the elimination of bromine as acetylbromide to 5-methyl-3-[P-(3'aryl-acryl-1'-oyl)-phenyl]-3H-Δ4-2-oxo-1,3,4-oxadiazole 7a-d. Nucleophilic addition of NH2 group of thiourea on βcarbon of 7a-d in presence of a base, followed by intermolecular dehydrative cyclisation,afforded the final product 5-methyl-3-[P-(6'-aryl-2'-thioxo-1',2',5',6'-tetrahydropyrimidin-4'-yl)-phenyl]-3H-2- oxo-Δ4-1,3,4-oxadiazole 8a-d.
Melting points were determined using Thomas Hoover capillary melting point apparatus, and are uncorrected. IR spectra were recorded in KBr on Nicolet Impact - 410 FT -IR spectrophotometer. 1H NMR spectra were recorded on Bruker – 300 MHz FT-NMR spectrometer at 300 MHz. The chemical shifts are reported as parts per million relative to internal standard TMS. Elemental analyses results are within 0.4% of the calculated value. TLC was performed on preactivated (110°) silica gel plates, using acetone-methanol (6:4) as irrigant. Compounds 1, 3a-d, 4ad, 5 and 6a-d were prepared according to the literature. [16] The compound 7a-d and the title compound 8a-d, were prepared using the following procedures.
Compound 6a (0.01 mol) was suspended in pure acetic anhydride (5 ml), and the mixture was heated at 140° on oil bath, until the brown fumes of acetyl bromide were removed (2 h). The mixture was poured to water, and allowed to stand for 30 min. The yellow coloured solid was filtered and washed with water, and recrystallised using methanol-dioxane, to get bright yellow crystals of 7a. Similarly, 7b-d were prepared.
A mixture of 7a (0.01 mol) and thiourea (0.01 mol) in ethanolic sodium hydroxide solution (10 ml, 10%), was refluxed for 4 h, cooled, and poured into ice cold dil HCl. The separated solid was filtered off, washed with cold water to get 8a, and recrystallised using absolute ethanol. Similarly, compounds 8b-d were prepared (Table 1).
| Compound | Mol. formula | Analysis found (Calcd.) % | Yield% | m.p.° | Rf | ||||
|---|---|---|---|---|---|---|---|---|---|
| C | H | N | |||||||
| 7a | C18 H14 N2 O3 | 70.56 | (70.58) | 4.63(4.61) | 9.17 | (9.15) | 72 | 137-9 | 0.46 |
| 7b | C18 H13BrN2 O3 | 56.10 | (56.12) | 3.38(3.40) | 7.24 | (7.27) | 70 | 182-4 | 0.31 |
| 7c | C19 H16 N2 O3 | 71.29 | (71.24) | 5.04(5.03) | 8.72 | (8.74) | 75 | 125-7 | 0.49 |
| 7d | C18H13ClN2O3 | 63.42 | (63.44) | 3.84(3.85) | 8.24 | (8.22) | 70 | 196-8 | 0.40 |
| 8a | C19 H16 N4 O2 S | 62.60 | (62.62) | 4.41(4.43) | 15.35 | (15.37) | 88 | 112-4 | 0.50 |
| 8b | C19H15 BrN4 O2 S | 51.46 | (51.48) | 3.39(3.41) | 12.66 | (12.64) | 80 | 174-6 | 0.38 |
| 8c | C20 H18N4 O2 S | 63.45 | (63.47) | 4.77(4.79) | 14.77 | (14.80) | 86 | 135-7 | 0.57 |
| 8d | C19 H15 ClN4O2S | 57.20 | (57.21) | 3.80(3.79) | 14.07 | (14.05) | 85 | 201-3 | 0.65 |
Compounds 7a-d were recrystallised from methanol-dioxane mixture (1:1) and 8a-d from absolute ethanol.
Table 1: The characterisation data
The compounds 7a-d showed IR absorptions in the range 1652-1658 cm-1, corresponding to α, β-unsaturated carbonyl group. Another sharp band appeared in the range 1772-1793 cm-1, due to lactone carbonyl function. 1H NMR spectral studies have shown a singlet at δ 2.052.14, assigned to C5 methyl protons. Two doublets appeared due to C3 and C2 vinylic protons. Aromatic protons appeared as multiplets in the region 7.07-8.01 ppm. The title compounds 8a-d were also confirmed by their IR and 1H NMR spectral studies. The compounds 8a-d have shown IR absorption peaks in the region 1300-1328 cm-1 (C=S), 1594-1615 cm-1 (C=N), 1769-1781 cm-1 (C=O), and a broad band due to N-H stretching in the region 3309-3421 cm-1. 1H NMR spectral studies of 8a-d, exhibited a singlet at δ 1.95-2.14 due to C5 methyl protons, the diastereotopic protons HA and HB appeared as two doublets in the range 3.36-3.90. A multiplet was observed at 4.61-5.00, assigned to C6 proton, and at 4.955.54
a broad singlet,due to N-H proton (D2O exchanged), which appeared. All the aromatic protons appeared in the range 7.10-7.78 ppm.
Antibacterial activity of newly synthesised compounds was determined against Staphylococcus aureus and Pseudomonas aeruginosa, by the cup plate method. [17] The test compounds were dissolved in dimethyl formamide, and different aliquots were placed in each cup. Incubation was carried out at 37° for 24 h. The diameter of zone of inhibition was measured for 100 μg/ml concentration.
The antifungal activity of the compounds was evaluated against Aspergillus flavus and Fusarium oxysporium, by adopting the cup plate technique. The diameter of zone of inhibition was measured for 100 μg/ml concentration. The results of antibacterial and antifungal activities are recorded in Table 2.
| Compound | Diameter of zone of inhibition (mm)* | |||
|---|---|---|---|---|
| S. aureus | P. aeruginosa | A. flavus | F. oxysporium | |
| 8a | 14.0 | 13.0 | 12.5 | 14.0 |
| 8b | 22.0 | 23.0 | 20.0 | 17.0 |
| 8c | 15.5 | 14.8 | 15.0 | 11.0 |
| 8d | 20.0 | 21.0 | 24.0 | 15.0 |
| Norfloxacin | 26.0 | 25.0 | - | - |
| Griseofulvin | - | - | 28.0 | 25.0 |
*Size of the inhibition zone by disk diffusion method, Control (DMF) = No activity, Both test compounds and standards were tested at 100 g/ml conc.
Table 2: Results of in vitro antimicrobial activity.
In case of antibacterial and antifungal activities, the standard drugs used were norfloxacin and griseofulvin respectively, because these drugs and the newly synthesized compounds are easily soluble in DMF solvent. Hence, it was advantageous to use these drugs as standard. Apart from this, griseofulvin is less toxic, and MIC values for norfloxacin are higher.
Antiinflammatory activity was measured using the carrageenan-induced paw edema test in rats. [18] Commercially available ibuprofen was used as a standard drug, because it is well absorbed, and can bind easily to plasma proteins. Male Swiss rats (150-200 g) were divided into control, standard, and test groups, each consisting of six rats. All protocols of animal experiments have been approved by the Institutional Animal Ethics Committee (IAEC). A group of rats was treated with Tween-80 (1%) suspension i.p. (control). Another group was treated with a dosage of 100 mg/kg of the suspension of the test compounds. After 30 min, the animals were injected with
0.1 ml of carrageenan (1% w/v), in the sub plantar region of left hind paw of the rats. The volume of the paw was measured using the mercury displacement technique, with the help of a plethysmograph, both in control as well as in standard animals, including the test animals 2 h and 4 h, after injection. The initial volume of the paw was measured within 30 sec of the injection. The percent inhibition of the inflammation after 2 h and 4 h, was calculated using the relation, % inhibition = (1-vt/vc)×100, where, vt and vc are the mean relative changes in the volume of paw edema in the test and control, respectively. The results are summarised in Table 3.
| Compound | Dose mg/kg | Edema volume (ml)x Different interval (mean±S.E.M) | % Inhibition | ||
|---|---|---|---|---|---|
| 2h | 4h | 2h | 4h | ||
| 8a | 100 | 0.20±0.02 | 0.26±0.03 | 55.6 | 42.2 |
| 8b | 100 | 0.40±0.02 | 0.39±0.02 | 11.2 | 13.0 |
| 8c | 100 | 0.28±0.02 | 0.28±0.03 | 37.7 | 38.0 |
| 8d | 100 | 0.38±0.02 | 0.41±0.03 | 15.6 | 08.9 |
| Standard (Ibuprofen) | 100 | 0.30±0.03 | 0.30±0.02 | 33.4 | 33.4 |
| Control (Tween) | - | 0.45±0.03 | 0.45±0.02 | - | |
x Values are mean±S.E.M, no.of animals in each group is 06.
Table 3: Results of antiinflammatory activity
The anticonvulsant activity of title compounds was based on maximal electroshock-induced convulsions in rats. [19] Male Swiss rats were procured from the Virus Diagnostic Laboratory, Dharwad, and maintained at Department of Studies in Botany, Karnataka University Dharwad, They were fed with standard diet, water, and libitum. Six groups of three rats were selected, and to the first group, saline (control) was injected i.p., placed corneal electrodes were placed on the cornea, followed by application of the prescribed current. The different stages of convulsions were noted, and used as control. Phenytoin sodium was used as standard, because it is not a CNS depressant, but some sedation may occur at therapeutic dose The second group was treated with a dosage of 25 mg/kg of phenytoin sodium in the form of injection i.p., and after 30 min, was subjected to electroconvulsions. The same procedure was repeated for the remaining four groups, using test compounds 8a-d. Various stages of convulsions were recorded at different intervals. The mean value for each group was calculated and compared with control. The results are summarised in Table 4.
| Compound | Dosea mg/kg | Time (sec) in various phases of convulsion | Recovery/death | |||
|---|---|---|---|---|---|---|
| Flexion | Extensor | Clonus | Stupor | |||
| 8a | 25 | 3.3 | 5.0 | 2.5 | 86 | Recovery |
| 8b | 25 | 3.1 | 4.5 | 2.4 | 83 | Recovery |
| 8c | 25 | 1.6 | 4.0 | 2.3 | 92 | Recovery |
| 8d | 25 | 2.5 | 3.8 | 2.2 | 94 | Recovery |
| Control (Saline) | - | 4.0 | 11.0 | 3.0 | 120 | Recovery |
| Standard (Phenytoin) | 25 | 1.2 | 2.0 | 0.9 | 100 | Recovery |
a No. of animals in each group is 06.
Table 4: Results of anticonvulsant activity
From the antibacterial results (Table 2), it was observed that bromo and chloro substituted compounds 8b and 8d were more active than 8a and 8c. A similar trend can also be observed in the antifungal activity data. These results imply, that the presence of bromo and chloro substituents might have enhanced the antimicrobial activity. The antiinflammatory activity (Table 3), revealed that the compounds 8a (55.6%) and 8c (37.7%), showed activity more than the standard drug. Compounds 8b and 8d have shown weak activity. Amongst the compounds subjected to anticonvulsant activity (Table 4), compounds 8c and 8d were found to possess promising activity, compared to that of standard phenytoin.
Thus, the synthesis described herein, provides oxadiazole derivatives 8a-d in good yields, which have shown promising biological activities. The method adopted in this paper is simple, efficient, environment friendly, inexpensive, and it is useful in constructing pharmacologically important heterocycles.
Acknowledgements
Authors are thankful to Dr. Ravi S. Chavan, Dept. of studies in Botany, Karnatak University Dharwad for carrying out pharmacological activities.
References
- Yelamaggad, C.V., Hiremath, U.S. and Badami, B.V., Indian J.Chem., 1994, 33B, 674.
- Patil, B.M., Badami, B.V. and Puranik, G.S., Indian Drugs., 1995, 32, 439.
- Kavali, J.R., Badami, B.V., Il Farmaco., 2000, 55, 406.
- Kavali, J.R., Badami, B.V., J. Chem. Res. (s) and (m)., 2000, 546.
- Mallur, S.G., Badami, B.V., Indian J. Chem., 2001, 40B, 742.
- Ohta, M., and Kato, H., In; Snyder, J.P., Ed., Non Benzenoid Aromatics, Academic Press, New York, 1969, 117.
- Mallur, S.G., Badami, B.V., Il Farmaco., 2000, 55, 65.
- Rupe, H., Gebhardt, H., Berichte., 1974, 32, 671.
- Su, T.L., Watanabe, K.A., J. Org. Chem., 1989, 54, 220.
- Gupta, A., Sharma, R and Prakash, L., J. Indian Chem. Soc., 1994, 71, 635.
- Inoue, M., Hashimoto, K., Jp patent No., Jp03204877, 1991.
- Mogliah, K., Chowdary, D.S. and Rao, R.B., Indian J. Chem., 2001, 40B, 43.
- Shah, V.R., Vadodaria, M. and Parikh, A.R., Indian J. Chem., 1997, 36B, 100.
- Ladva, K., Patel, P., Upadhyay, P. and Parekh, H., Indian J.Chem., 1996, 35B, 1062.
- Su, X.W., Hui, X.P., Chu, C.H. and Zhang, Z.Y., Indian J. Chem., 2001, 40B, 15.
- Dambal, D.B., Badami, B.V and Puranik, G.S., Indian J. Chem., 1982, 21B, 865.
- Saundane, A.R., Rudresh, K., Satyanarayana, N.D and Hiremath, S.O., Indian J. Pharm. Sci., 1998, 60, 379.
- Misra, A.K., Dandia, P.C. and Kulkarni, S.K., Indian J.Pharmacol., 1973, 5, 449.
- Winter, C.A., Risley, E.A., and Nus, G.N., Proc. Soc. Exp. Biol., 1962, 111, 544.