- Corresponding Author:
- L. Talesara
Synthetic Organic Chemistry Laboratory, Department of Chemistry, M. L. Sukhadia University, Udaipur-313001, India.
E-mail: gtalesara@yahoo.com
| Date of Submission | 15 January 2007 |
| Date of Revision | 7 April 2006 |
| Date of Acceptance | 5 December 2005 |
| Indian J Pharm Sci, 2007, 69 (1): 28-32 |
Abstract
Arylamines on diazotization and further treatment with dicyandiamide yielded aryldicyandiamide (1a-d), which on addition with aminoxy compound (2) gave corresponding biguanides (3a-d). Cycloaddition of biguanide with ethylchloroacetate furnished 2,4,6-trisubstituted-s-triazines (4a-d). Subsequent treatment of these compounds with N-hydroxyphthalimide or N-hydroxysuccinimide in presence of triethylamine gave final compounds (5a-h). IR, 1 H NMR and mass spectra were used to confirm their structure. Compounds (5a-h) were screened for antibacterial ( Escherichia coli, Proteus vulgaris, Klebsiella pneumoniae, Pseudomonas aureus and Staphylococcus aureus ) and antifungal ( Candida albicans and Aspergillus fumigatus ) activities. Antibacterial activity revealed that compounds 5a, 5b, 5c, 5g showed comparable activity against bacteria P. vulgaris, P. aureus, where rest of the compounds showed weak activity against all the pathogenic bacteria. The fungicidal data indicated that compound 5d possess high level activity and rest of the compounds showed comparable to the standard.
In the present communication, a series of 2-(2',4'-dinitroarylaminoxy)-4-methoxyphthalimido or methoxysuccinimido-6-(4"-subs.anilino)-s-triazine (5a-h) have been synthesized and tested for antimicrobial activities. The synthesis of s-triazine [1] and their pharmacological applications [2-5] are well documented. Some 2-arylamino-4-chloro-6-(pyrimidine-4-carboxyhydrazino)-1,3,5-triazines have been synthesized6 and observed to possess remarkable antitubercular activity. Several derivatives of s-triazine show antimicrobial [7], antibacterial [8] and herbicidal [9] activities. These are also used for treatment of HIV infection [10]. Phthalimidoxy derivatives represent one of the most active class of compounds possessing biological activities [11,12]. N-phthalimidoxy-2-methacrylate, ethyl Nphthalimidoxy acetate containing phthalimidoxy group have been demonstrated to possess anticancer [13], anticonvulsant [14], antimalarial15, hypotensive16 and antiamoebic [17] properties. Aminoxy moiety has a special place in the modern therapy of malaria [18]. Synthesis and antimicrobial properties of substituted 3-aminooxy propionyl and 3-aminooxy (E)-2-methoxyimino propionyl monolactams have been reported [19]. Combination of all these three biological moieties in one molecule might result in the overall enhanced biological activity.
Materials and Methods
Melting points of synthesized compounds were determined in open capillary tubes and are therefore uncorrected. The structures of compounds were established on the basis of elemental analysis and spectral data. The IR spectra were recorded in the range of 4000-450 cm-1 using KBr pellets on a FTIR RXI Perkin-Elmer spectrophotometer. 1H NMR spectra were recorded on a Bruker DRX 300 MHz spectrophotometer using CDCl3/ DMSO-d6 as solvent. The FAB mass spectra were recorded on a Jeol SX-102/DA-6000 spectrometer data system using argon/xenon (6KV,10 mA) as FAB gas. Purity of synthesized compounds was checked by silica gel-G plates of 2 mm thickness using benzene and ethyl acetate (9:1 and 1:9) as solvent system and iodine chamber as developer. N-Hydroxyphthalimide was prepared by reported method [20].
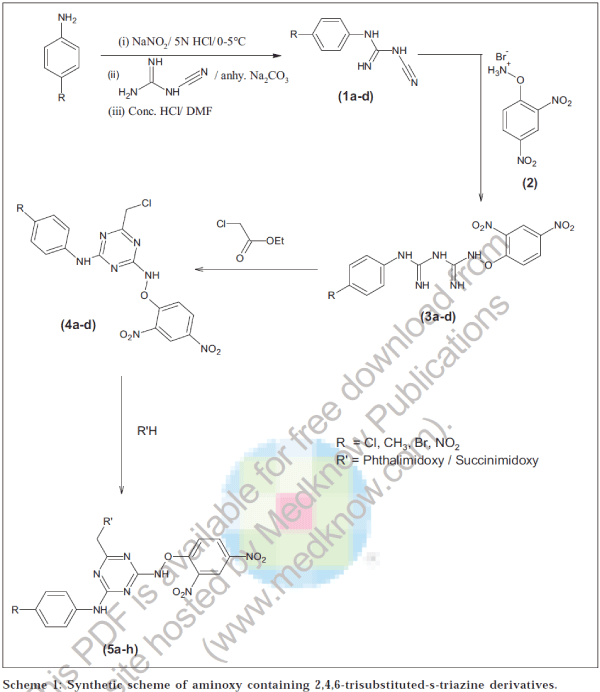
4-Substituted aryl dicyandiamide (1a-d)
Suspension of p-substituted aniline (0.01 mol) in hot 5 N HCl was cooled to 0-5°. It was diazotized by adding sodium nitrite (0.01 mol) solution in water, whether the reaction has completed or not was checked by starch-iodide paper [21]. Diazotized mixture was slowly added to a solution of dicyandiamide (0.01 mol) in water (25 ml) with stirring, maintaining the temperature at 0-5°. Anhydrous sodium carbonate was added in small quantities during 2 h to maintain alkalinity of the reaction. Triazine obtained was washed with water and dried. This was then added during half an hour to a stirred mixture of DMF (50 ml) and 10 N HCl (20 ml) at 30-35°. This reaction mixture was further stirred for half an hour. Water was added to obtain a precipitate. Crude product was crystallized from methanol. Similarly other compounds (1b-d) were prepared.
2,4-Dinitrophenoxyamine hydrochloride (2)
2,4-Dinitrochlorobenzene (0.01 mol) in DMF was added to a stirred solution of N-hydroxypthalimide (0.01 mol) in CHDMF. Reaction mixture was stirred at room temperature for 5-6 h and then left for overnight. It was filtered and the filtrate was poured in ice cold water. Product 2,4-dinitrophenoxyphthalimide(2) obtained was recrystallized from alcohol and hydrolyzed by dissolving it in glacial acetic acid and boiled with hydrobromic acid (37%) for 20-30 min. Phthalic acid separated on cooling was filtered, solvent was removed under reduced pressure. Crude CHsolid was washed with petroleum ether.
N1-(2,4-Dinitroaryloxy)-N5-(4'-substituted aryl) biguanides (3a-d)
A mixture of (1a, 0.01 mol) was refluxed with (2, 0.01 mol) in absolute alcohol for 7-8 h. It was filtered and the filtrate was diluted with water. Sodium bicarbonate was added to it till turbidity appears. It was left for 10 h at room temperature. Crude product obtained was recrystallized from dilute methanol. Other compounds (3 b-d) were prepared by similar method with slight variation in refluxing time.
2-(2',4'-Dinitroaryloxyamino)-4-(chloromethyl)-6-d(4"-substituted anilino)-s-triazine (4a-d)
To a stirred solution of (3a, 0.01 mol) in methanol, sodium (0.01 mol) was added. It was further stirred for 20 min, after this ethylchloroacetate (0.01 mol) was added. It was stirred further for 24 h [22]. After evaporation of the solvent and recrystallization from absolute alcohol, yellow-brown crystals were obtained. Similarly other compounds (4b-d) were also prepared with minor modification in mole ratio of reagents and stirring period.
2-(2',4'-Dinitroaryloxyamino)-4-methoxyphthalimido or methoxysuccinimido-6-(4"-substituted anilino)-striazine (5a-h)
To a stirred solution of (4a, 0.01 mol) in DMF, a solution of N-hydroxyphthalimide (0.01 mol) in DMF was added in presence of TEA (0.02 mol) as a base. It was further stirred for 24 h. It was filtered and the filtrate was poured in crushed ice. Crude product obtained was recrystallized from absolute alcohol. Compounds (5b-h) were also synthesized by similar method using reagents in proper mole ratio. The entire synthetic route has been shown in scheme 1.
Characteristics of 5a
IR (KBr, νmax in cm-1) : 3455 (NH str.), 3080 (Ar-H), 2892 (CH2), 1700 (CO-N-CO), 1615 (C=N), 1510, 1300 (NO2), 1280 (C-N), 1120 (C-O), 980 (N-O), 740 (C-Cl); PMR (DMSO-d6, δ in ppm): 8.0 (m, 3H, Ar-H, near NO2), 7.4 (d, 2H, Ar-H, near Cl), 7.1-6.9 (m, 6H, Ar-H), 6.7 (s, 1H, NH-O), 4.8 (s, 1H, NH), 3.6 (s, 2H, CH2-O); m/z : 580 [M+2]+•, 578 [M]+•, 395, 268, 122, 106, 92, 66, 40.
Characteristics of 5b
IR (KBr, νmax in cm-1) : 3471 (NH str.), 3067 (Ar-H), 2960 (CH3), 2874 (CH2), 1712 (CO-N-CO), 1603 (C=N),1500, 1399 (NO2), 1121 (C-O), 935 (N-O); PMR (DMSO-d6 , δ in ppm): (m, 3H, Ar-H, near NO2), 7.1 (m, 8H, Ar-H), 6.7 (s, 1H, NH-O), 4.8 (s, 1H, NH), 3.5 (s, 2H, CH2-O), 2.2 (s, 3H, CH3 ); m/z : 558 [M]+•, 375, 268, 122, 106, 66, 40.
Characteristics of 5c
IR (KBr, νmax in cm-1) : 3420 (NH str.), 3085 (Ar-H), 2888 (CH2), 1708 (CO-N-CO), 1610 (C=N), 1510 (NO2), 1120 (C-O), 995 (N-O), 580 (C-Br); PMR (DMSO-d6, δ in ppm): 8.0 (m, 3H, Ar-H, near NO2), 7.2 (m, 8H, Ar-H), 6.7 (s, 1H, NH-O), 4.8 (s, 1H, NH), 3.6 (s, 2H, CH2-O); m/z : 614 [M+1]+•, 613 [M]+•, 449, 268, 122, 100, 40.
Characteristics of 5d
IR (KBr, νmax in cm-1) : 3450 (NH str.), 3090 (Ar-H), 2890 (CH2), 1690 (CO-N-CO), 1675 (C=N), 1510, 1300 (NO2), 1275 (C-N), 1120 (C-O), 920 (N-O); PMR (DMSO-d6, δ in ppm): 8.1 (m, 7H, Ar-H, near NO2), 7.1 (m, 4H, Ar-H), 6.7 (s, 1H, NH-O), 4.9 (s, 1H, NH), 3.6 (s, 1H, CH2-O); m/z : 589 [M]+•, 406, 268, 122, 106, 66, 40.
Characteristics of 5e
IR (KBr, νmax in cm-1) : 3445 (NH), 3080 (Ar-H), 2895 (CH2), 1680 (CO-N-CO), 1625 (C=N), 1500 (NO2), 750 (C-Cl); PMR (DMSO-d6, δ in ppm): 8.0 (m, 3H, Ar-H, near NO2), 7.4 (d, 2H, Ar-H, near Cl), 7.0 (d, 2H, Ar-H, near NH), 6.8 (s, 1H, NH-O), 4.8 (s, 1H, NH), 3.5 (s, 1H, CH2O), 2.2 (t, 4H, CH2-CO); m/z : 532 [M+2]+•, 530 [M]+•, 432, 416, 402, 275, 92, 78, 52, 26.
Characteristics of 5f
IR (KBr, νmax in cm-1) : 3440 (NH), 2990 (CH3), 2880 (CH2), 1670 (CO-N-CO), 1610 (C=N), 1500 (NO2); PMR (DMSO-d6, δ in ppm): 8.0 (m, 3H, Ar-H, near NO2), 7.2 (m, 4H, Ar-H), 6.7 (s, 1H, NH-O), 4.7 (s, 1H, NH), 3.5 (s, 1H, CH2-O), 2.3 (s, 3H, CH3), 2.2 (t, 4H, CH2); m/z : 510 [M]+•, 412, 396, 382, 275, 92, 78.
Characteristics of 5g
IR (KBr, νmax in cm-1) : 3430 (NH), 2890 (CH2), 1690 (CO-N-CO), 1630 (C=N), 1510 (NO2), 550 (C-Br); PMR (DMSO-d6, δ in ppm): 8.1 (m, 3H, Ar-H, near NO2), 7.2 (m, 4H, Ar-H), 6.7 (s, 1H, NH-O), 4.6 (s, 1H, NH), 3.6 (s, 1H, CH2-O), 2.3 (t, 4H, CH2); m/z : 575 [M+1]+•, 574 [M]+•, 476, 460, 446, 275, 92, 26.
Characteristics of 5h
IR (KBr, νmax in cm-1) : 4450 (NH), 2892 (CH2), 1695 (CO-N-CO), 1638 (C=N), 1500, 1310 (NO2); PMR (DMSOd6, δ in ppm): 8.2 (m, 7H, Ar-H, near NO2), 6.9 (s, 1H, NH-O), 4.8 (s, 1H, NH), 3.8 (s, 1H, CH2-O), 2.4 (t, 4H, CH2); m/z : 541 [M]+•, 443,427, 413, 275, 78.
Antimicrobial activity.
Antimicrobial activity was assayed by well or cup method [23] in nutrient agar and Sabourand dextrose agar. Media was inoculated with 0.2 ml suspension of organisms by spread plate method [24]. With the help of sterile borer, a well was made in the centre of medium and filled with 300 μg/ml concentration of synthesized compounds. The incubation time was 24 h at 37° for bacteria and 74 h at 37° for fungal strains. Antimicrobial activity was measured as a function of diameter of zone of inhibition (mm). The results were compared to amicacin and levofloxacin for antibacterial activity and fluconazole for antifungal activity by measuring zone of inhibition in mm at 300 μg/ml concentration using disc diffusion method [25].
Results and Discussion
Diazotization of 4-substituted arylamine in presence of NaNO2 and 5N HCl at 0-5° and subsequent treatment of dicyandiamide in alkaline medium yielded 4-substituted aryl dicyandiamide (1a-d). The formation of compound (1a) was confirmed on the basis of spectral data. IR data reveals the presence of NH stretching at 3450, C≡N stretching at 2250 and C=N stretching at 1680 cm-1. Furthermore, the presence of singlet at δ 4.5 ppm of NH confirmed the formation of compound (1a). Compounds (1a-d) when treated with aminoxy compound (2) in alcoholic medium yielded corresponding biguanides (3a- d). Formation of compound (3a) was confirmed by the disappearance of C≡N peak at 2250 cm-1 and appearance of NO2 peak at 1500, 1350 cm-1 and multiplet at δ 8.0 ppm (DMSO) for the aryl proton near NO2. Cycloaddition of compounds (3a-d) with ethylchloroacetate in presence of sodium furnished 2,4,6-trisubstituted-s-triazine (4a-d). Structure of 4a was confirmed by the appearance of C-Cl peak at 740 and CH2 at 2890 cm-1. Compounds (4a-d) were lastly condensed with N-hydroxyphthalimide or N- hydroxysuccinimide in presence of TEA to obtain final compounds (5a-h). Disappearance of C-Cl peak at 740 and appearance of CO-N-CO peak at 1712 cm-1 confirmed the structure of compounds (5a-h). Melting points, yields and elemental analysis of these compounds are given in Table 1.
| Compd. | R | R’ | Mol. Formula/Mol. Wt. | mp (0) | Yield (%) | % of N Calc./ found |
|---|---|---|---|---|---|---|
| 1a | Cl | - | C8H7N4Cl/194.5 | 201 | 60 | 28.79/28.52 |
| 1b | CH3 | - | C9H10N4/174 | 211 | 75 | 32.18/31.90 |
| 1c | Br | - | C8H7N4Br/229 | 219 | 50 | 24.45/23.10 |
| 1d | NO2 | - | C8H7N5O2/205 | 245 | 67 | 34.14/34.00 |
| 2 | - | - | C6H5N3O5/199 | 120 | 40 | 21.10/21.05 |
| 3a | C1 | - | C14H12N7O5C1/393.5 | 234 | 50 | 24.90/24.82 |
| 3b | CH3 | - | C15H15N7O5/373 | 220 | 70 | 26.27/26.10 |
| 3c | Br | - | C14H12N7 O 5Br/428 | 198 | 35 | 22.89/22.24 |
| 3d | NO2 | - | C14H12N8O 7/404 | 208 | 25 | 27.72/27.52 |
| 4a | C1 | - | C16H 11N 7O 5 C1 2/451 | 196 | 40 | 21.72/21.50 |
| 4b | CH3 | - | C17H14N7O5 C1/431.5 | 200 | 45 | 22.71/22.65 |
| 4c | Br | - | C16H11N7 O5C1 Br/496.5 | 210 | 40 | 19.73/19.62 |
| 4d | NO 2 | - | C16H11N8O7 C1/462.5 | 190 | 32 | 24.21/24.15 |
| 5a | C1 | Phthali-midoxy | C24H15N8O8 C1/578.5 | 222 | 40 | 19.36/19.25 |
| 5b | CH3 | " | C25H18N8O8/558 | 256 | 45 | 20.07/19.68 |
| 5c | Br | " | C24H15N8O8 Br/614 | 238 | 30 | 18.27/17.65 |
| 5d | NO2 | " | C24H15N9O10/589 | 164 | 28 | 21.39/19.00 |
| 5e | C1 | Succini-midoxy | C20H15N8O 8C1/530.5 | 198 | 42 | 21.11/21.01 |
| 5f | CH3 | " | C21H18N8O8/510 | 208 | 40 | 21.96/21.94 |
| 5g | Br | " | C20H15N8O8Br/575 | 188 | 32 | 19.47/19.40 |
| 5h | NO2 | " | C20H15N9O10/541 | 170 | 30 | 20.70/20.68 |
Table 1: Physical and Analytical Data of Synthesized Compounds
The synthesized compounds (5a-h) were tested in vitro for antibacterial and antifungal activities against Escherichia coli, Proteus vulgaris, Klebsiella pneumoniae, Pseudomonas aureus, Staphylococcus aureus, Candida albicans and Aspergillus fumigatus (Table 2).
| Compounds | Antibacterial activity (300 µg/ml) | Antifungal activity (300 µg/ml) | ||||||
|---|---|---|---|---|---|---|---|---|
| E. coli | P. valgaris | K. pneumoniae | P. aureus | S. aureus | A. fumigatus | C. albicans | ||
| 5a. | 12 | 20 | 13 | 19 | 8 | 20 | 26 | |
| 5b. | 10.2 | 17 | 13.6 | 21 | 10 | 24 | 19 | |
| 5c. | 11 | 18 | 12 | 20 | 12 | 22 | 22 | |
| 5d. | 14 | 18.5 | 14 | 17 | 9 | 26 | 20 | |
| 5e. | 9 | 15 | 12.2 | 14 | 7 | 21 | 20 | |
| 5f. | 13.5 | 18 | 15 | 18 | 8.2 | 23 | 21 | |
| 5g. | 14 | 17 | 12 | 20 | 6 | 20 | 23 | |
| 5h. | 12 | 13 | 12.5 | 15 | 8 | 19.5 | 25 | |
| Amicacin* | 23 | 21 | 23 | 21 | 28 | - | - | |
| Levoflaxacin* | 21 | 23 | 26 | 23 | 25 | - | - | |
| Fluconazole* | - | - | - | - | - | 25 | 30 | |
Table 2: Results of Antimicrobial Studies of Compounds Zone of Growth Inhibition
The synthesized compounds at 300 μg/ml concentration showed low antibacterial activity as compared to the standard amicacin and levofloxacin while these also found to show good antifungal activity as compared to the standard fluconazole. All the compounds were found to be more active towards Aspergillus fumigatus than Candida albicans. It can be concluded from antimicrobial activity that combination of all the three biological moieties i.e., phthalimidoxy, aminoxy and s-triazine in one molecule results in the enhancement of biological activity.
Acknowledgements
The authors are thankful to Head, Department of Chemistry, M.L. Sukhadia University, Udaipur. for providing laboratory facilities and Head, Department of Botany, M.L. Sukhadia University, Udaipur for antimicrobial studies. Authors are also thankful to the Director, RSIC, CDRI, Lucknow for spectral and analytical studies. One of the authors (DB) is thankful to UGC, New Delhi for providing necessary financial assistance.
References
- Smolin, E.M., In; Raport, L., s-Triazine and derivatives, Interscience, New York, 1959, 129.
- Burger, A., In; Medicinal Chemistry, Interscience, New York, 1970, 1338.
- Shabadi, C.V., Shelar, M.A. and Shelar, A.R., Indian Drugs, 1998, 35, 488.
- Brzozowski, Z., Chem. Abstr., 1998, 129, 260430u.
- Kreutzberger, A. and Rose O., Chem. Abstr., 1987, 108, 131766a.
- 6.Dave, M.P. and Thaker, A.K., J. Indian Chem. Soc., 1984, 61, 237.
- Desai, P.S. and Desai, K.R., J. Indian Chem. Soc., 1994, 77, 155.
- Gajare, A.S. and Shingare, M.S., Indian J. Chem., 1998, 37B, 510.
- Nishimura, N. and Kato, A., Carbohyd. Res., 2001, 331,77.
- Kukla, M.J. and Janssen, P.A., J. Eur. Pat., 1999, 447, 945.
- Mamalis, P., Xenobiotica, 1971, 45, 569.
- Ure, J.A. and Perassolo, M., J. Neuro Sci., 2000, 1, 177.
- Farrar, Y.A., Rutkowska, M.C., Grochowski, J., Serda, P., Pilati, T. Cary, M. and Scott, K.R., J. Med. Chem. , 1993, 36, 3517.
- Lanar, G.D., Owoyale, J.A., Edafiogno, I.O. and Osuice, G. Pharmacy World J.,, 1986, 5, 307.
- Venugopalan, B., Satlue, K.M. and Chatterjee, D.K., Indian J. Chem., 1995, 34B, 778.
- Sudan, S., Gupta, R., Rahishwara, Singh, G.B., Bania, S. and Kachroo, P.L., Indian J. Chem., 1995, 34B, 458.
- Asthana, P. and Rastogi, S.N., Indian J. Chem., 1987, 26B, 330.
- Berger, B.J., Antimalarial Agent Chemother., 2000, 44, 2590.
- Macchia, B., Rossela, A. and Broccali, G., Pharmacol., 1990, 49, 879.
- Orndrroff, W.R. and Pratt, D.S., Amer. J. Chem., 1912, 47, 89.
- Vogel, A.I., A Text book of Practical Organic Chemistry, 5th Edn. ELBS publishers, 1989, 921.
- Overberger, C.G. and Michelotti F.W., Organic Synthesis, Collective Volume IV, 1963, 29.
- Collee, G.J., Fraser, G.A., Marmion, P.B. and Simmons, A., Practical Medical Microbiology 14th Edn. Vol. 11, Churchill Livingstone, Edinburg, 1996, 163.
- Khalafallar, A.K., Selim, M.A., Abu Ethamd, R.M., Elamaghraby, M.A., Soleman, H.A. and Rasian, M.A., Indian J. Chem., 1995, 34B, 1066.
- Bisen, P.S. and Verma, K., In; Hand Book of Microbiology, 1st Edn.,CBS Publishers and Distributors, New Delhi, 1996, 30.