- *Corresponding Author:
- D. X. Zhao
School of Chemistry and Chemical Engineering, Henan University of Technology, Zhengzhou 450 001, China
E-mail: zhaodx798@163.com
| Date of Submission | 19 January 2016 |
| Date of Revision | 11 October 2016 |
| Date of Acceptance | 20 October 2016 |
| Indian J Pharm Sci 2016; 78(5): 688-691 |
This is an open access article distributed under terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Tranexamic acid derivatives modified by amino acids, i.e., tranexamic acid-His, tranexamic acid-Leu-His, tranexamic acid-Glu-Phe, tranexamic acid-Glu-Lys, tranexamic acid-Ser-Tyrand, tranexamic acid-Ser-Phe, were synthesized by Fmoc solid-phase peptide synthesis, purified by reversed-phase high performance liquid chromatography and characterized by 1H and electrospray ionisation mass spectrometry. The interactions of tranexamic acid and derivatives with ctDNA were preliminarily investigated by ultraviolet-visible absorption spectroscopy. The adsorption spectra of ctDNA show a hypochromic effect, indicating that tranexamic acid and derivative scan interact with ctDNA through a mixture mode of groove-binding and electrostatic interaction. The higher affinity of tranexamic acid derivatives for ctDNA compared with free tranexamic acid indicates that the side chains of amino acid residues helped form a special surrounding and spatial structure to interact with ctDNA. The stronger interaction of tranexamic acid derivatives with ctDNA suggested that the modified tranexamic acid probably had significant practical value and should be further studied.
Keywords
Tranexamic acid, derivative, synthesis, ctDNA, interaction
Tranexamic acid (TA) is often used for excessive bleeding by competitively inhibiting the activation of plasminogen to plasmin, a molecule responsible for the degradation of fibrin [1]. However, TA undergoes ionization in physiologic environments; its oral bioavailability is expected to be low due to inefficient absorption through membranes. There is a necessity to design and synthesize more lipophilic TA prodrugs. These prodrugs can provide the patent drug in a sustained release manner which might result in better clinical outcome, more convenient dosing regimens and less side effects than the original medication [2]. Many recent studies have focused on improving the stability, absorption and potency by peptidyl modification based on the peptide transporter system of mammalians, which can mediate the absorption of peptidyl drugs with amide bonds [3-5]. Tyrosine and histidine, have special functions for phenol and imidazole side chains in medicines, metalloproteins and specific enzymes by common coordinating ligands in vivo, aside from being protein frame. Glutamine, lysine, serine, phenylalanine and leucine are usually used as components of proteins to form a specific electrostatic and hydrophobic environment. Thus, tranexamic acid derivatives modified by amino acids, i.e. TA-His (1), TA-Leu- His (2), TA-Glu-Phe (3), TA-Glu-Lys (4), TA-Ser-Tyr (5) and TA-Ser-Phe (6) were efficiently synthesized in this study through solid-phase peptide synthesis and characterized by electrospray ionisation mass spectrometry (ESI-MS) and proton nuclear magnetic resonance (1H NMR). Preliminary studies on DNAbinding properties of derivatives using ultraviolet (UV) absorption spectroscopy are described.
Tranexamic acid, trifluoroacetic acid (TFA) and calf thymus DNA (ctDNA) were purchased from Sigma Co., Ltd. (Shanghai, China). Acetonitrile and methanol were purchased from Tianji Siyou Fine Chemicals Company (Tianjin, China). Fmoc- Tyr(tBu)-Wang resin, Fmoc-His(Trt)-Wang resin, Fmoc-Phe-Wang resin, Fmoc-Lys(Boc)-Wang resin, Fmoc-Ser(tBu)-OH, Fmoc-Leu-OH, Fmoc- Glu(tBu)-OH, Fmoc-Osu, N,N-diisopropylethylamine (DIEA), 1-hydroxybenzotriazolehydrate (HOBT), 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU) were purchased from GL Biochem Ltd. (Shanghai, China). Piperidine and triisopropylsilane (TIS) were purchased from Shanghai Crystal Pure Reagent Co. Ltd. (Shanghai, China), whereas ninhydrin, dimethylformamide (DMF), 1,4-dioxane and Na2CO3 were purchased form Tianjin Kemiou Co., Ltd. (Tianjin, China).
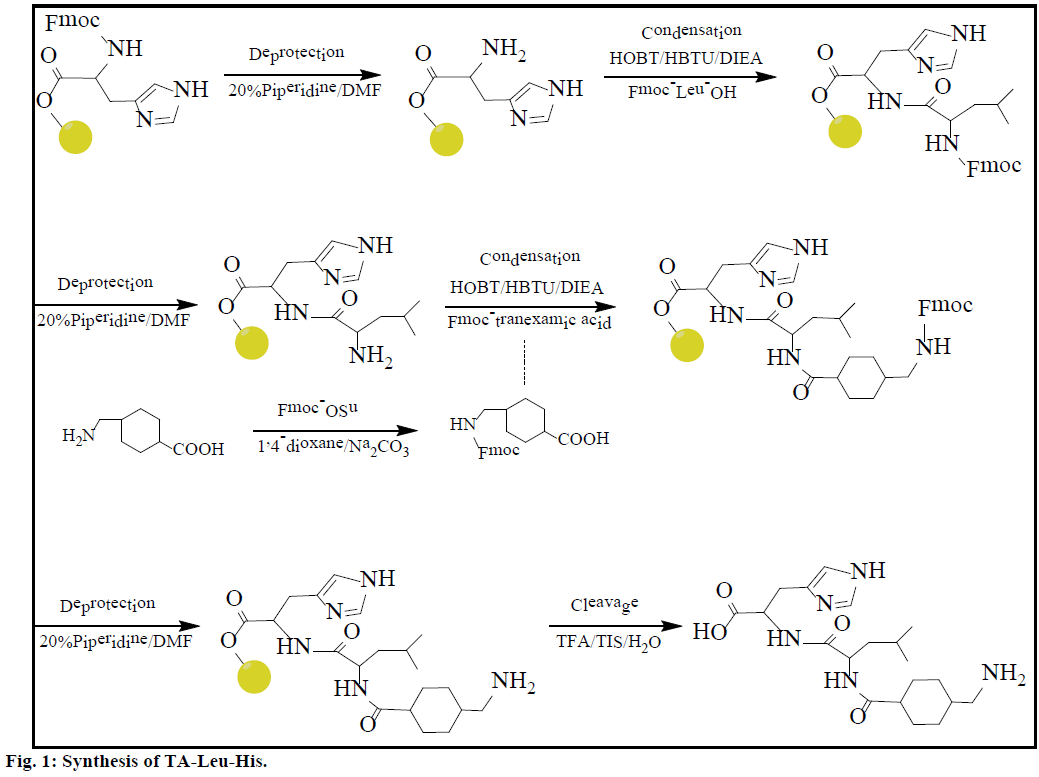
The synthesis process of 2 is shown in Figure 1 and detailed as follows [6]. Firstly TA was modified by Fmoc to Fmoc-TA [7-9]. 1,4-dioxane (20 ml) containing Fmoc- OSu (3.18 mmol) was added in 20 ml 10% Na2CO3 solution dissolved transamin 0.5 g (3.18 mmol) in the ice bath, and was stirred for 18 h at room temperature. The reaction solution was diluted with water, and extracted with 50 ml ether twice; the aqueous phase was acidified with HCl to pH 2.0 and cooled in an ice bath. After adding 50 ml ethyl acetate to extract the precipitate, the extraction was washed with water and saturated aqueous sodium chloride and dried with Na2SO4. The filtrate was concentrated under reduced pressure, crystallized by petroleum ether and filtered to obtain solid Fmoc-TA. The Fmoc-TA was dried (vacuum). Fmoc-His(Trt)-Wang Resin (0.35 mmol/g, 1.4286 g, 0.5 mmol) was placed in a peptide synthesis vessel and treated with DMF (10 ml, 30 min), 20% piperidine in DMF (10 ml, 5 min), 20% piperidine in DMF (10 ml, 60 min). The Kaiser test was performed at this point to identify the presence of free amine available for coupling. The resin was washed with CH3OH (3×8 ml, 5 min each) and DMF (3×8 ml, 5 min each). A coupling solution was prepared in a dried 50 ml round bottom flask by adding DIEA (0.413 ml) to a DMF (5 ml) solution that contains Fmoc-Leu-OH (0.365 g), HOBT (0.2714 g) and HBTU (0.7605 g) in an ice bath. The coupling solution was added to the peptide synthesis vessel containing the resin and the reaction was allowed to proceed for 6 h. The resin was washed with DMF (8×7.5 ml, 2 min each). After the Kaiser test showed full coupling, a coupling solution was prepared by adding DIEA (0.413 ml) to a DMF (5 ml) solution that contains Fmoc-TA (0.3522 g), HOBT (0.2714 g) and HBTU (0.7605 g) in an ice bath. This solution was added in the synthesis vessel and the reaction was allowed to proceed for 6 h. The resin was then washed with CH3OH (3×8 ml, 5 min each), and DMF (3×8 ml, 5 min each). The protective group in the coupled compound was removed by mixing with 20% piperidine in DMF (10 ml, 5 min) and 20% piperidine in DMF (10 ml, 60 min), and washing with CH3OH (3×8 ml, 5 min each) and DMF (3×8 ml, 5 min each). The cleavage of TA-Leu-His from the resin was accomplished using a 10 ml solution of 95% TFA, 2.5% TIS, and 2.5% water for 4 h. The syntheses of other derivatives are different depending on the amino acids used for coupling. The crude products were obtained by precipitation in cold diethyl ether. Purified derivatives were obtained via reversed phase high performance liquid chromatography (RP-HPLC).
TA derivatives were analyzed and purified on an Agilent C18 column (250×9.4 mm) with 5 μm silica as a stationary phase. A gradient elution with eluent A (water) and eluent B (acetonitrile) (30:70 v/v) was used at a flow rate of 1.0 ml×min-1. 1H NMR experiments were performed using a Bruker DPX-400 MHz instrument with TMS as the internal standard. Mass spectra were analyzed on an Agilent Esquire 3000 mass spectrometer fitted with an ion spray source working in positive ion mode and with methanol as the solvent. UV/Vis absorption spectra were recorded in a quartz cell (light path 10 mm) on a TU-1810 UV/Vis spectrometer. The ctDNA solution (3 ml, 3.75×10-3 mol×l-1) was added to the colorimetric ware and titrated with a specific volume of TA and derivatives (3.75×10-2 mol×l-1). The background absorption from all reagents was subtracted from the absorption spectra by using a corresponding solution without ctDNA as the reference solution.
Tranexamic acid modifiers were synthesized by solid phase peptide synthesis on Wang resin with good yield (~80%) and then analyzed via RP-HPLC. The final derivatives were isolated with high purity (>95%) and characterized via 1H-NMR and ESI-MS. The characterization results of TA derivatives are as follows:
Compound 1,1H NMR (400 MHz, D2O): δ8.53 (s, 1H, =CH), 7.20 (s, 1H, =CH), 3.05-3.10 (t, 1H, -CH), 2.70-2.80 (d, 2H, -CH2), 2.05-2.10 (s, 1H, -CH), 1.15- 1.35 (m, 2H, -CH2), 0.90-1.15 (m, 2H, -CH2). ESI/ MS: calcd for C14H22N4O3: 294.2. Found: m/z 295.2. Compound 2, 1H NMR (400 MHz, D2O): δ8.47-8.48 (d, 1H, -N=CH), 1.60-1.73 (m, 2H, -CH2), 1.31-1.54 (m, 2H, -CH2), 0.74-0.85 (m, 3H, -CH3). ESI/MS: calcd for C20H33N5O4: 407.4. Found: m/z 408.0. Compound 3, 1H NMR (400 MHz, D2O): δ7.15 (m, 1H, =CH), 4.52-4.56 (m, 1H, -CH), 4.09-4.13 (m, 1H, -CH), 2.70-2.72 (d, 2H, -CH2), 1.17-1.26 (m, 2H, -CH2), 0.87-0.96 (m, 2H, -CH2). ESI/MS: calcd for C22H32N3O6: 433.5. Found: m/z 434.0. Compound 4, 1H NMR (400 MHz, D2O): δ4.17-4.25 (m, 1H, -CH), 2.12-2.19 (m, 1H, -CH), 1.49-1.98 (m, 2H, -CH2), 1.23-1.36 (m, 2H, -CH2). ESI/MS: calcd for C19H34N2O4: 414.5. Found: m/z 415.0. Compound 5, 1H NMR (400 MHz, D2O): δ6.68- 6.72 (t, 1H, =CH), 6.98-7.03 (m, 1H, =CH), 4.19-4.27 (m, 1H, -CONH), 1.52-1.73 (m, 2H, -CH2), 1.16-1.26 (m, 2H, -CH2). ESI/MS: calcd for C20H29N3O7: 407.5. Found: m/z 407.9. Compound 6, 1H NMR (400 MHz, D2O): δ7.18-7.26 (m, 1H, =CH), 3.80-3.81 (d, 2H, -CH2), 1.50-1.54 (m, 2H, -CH2) and 1.21-1.31 (m, 2H, -CH2). ESI/MS: calcd for C20H29N3O6: 391.5. Found: m/z 392.0.
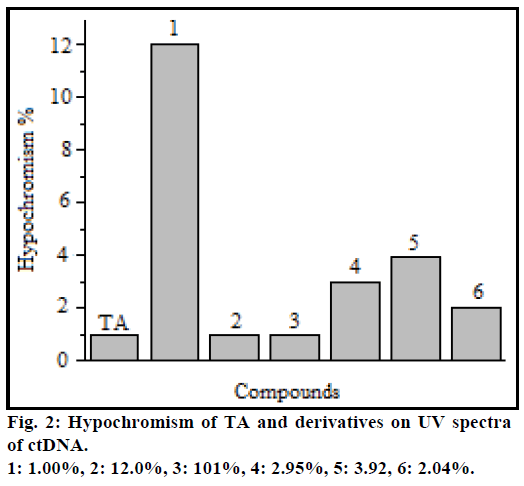
When small molecules interact with the DNA, the contraction and extension of the DNA molecule usually induces hypochromicity in UV spectra of the interaction system, and the explosion of DNA base pair lead hyperchromicity [10-12]. Ultraviolet absorption at 260 nm of ctDNA showed a hypochromic effect with increasing concentration of TA and derivatives and the maximum absorption wavelength remained unchanged. The hypochromism at maximum absorption wavelength in UV spectra of TA and derivatives is shown in Figure 2.
The difference in hypochromism of TA derivatives compared with that of free TA can be attributed to the side chains of amino acid residues which lead to the charge and spatial structure changes of derivatives, and affect the interaction [13]. The hypochromism of 1 to ctDNA was the largest which might be attributed to the imidazole of histidine residue, also the structure of 1 was easier to embed into the ctDNA groove than free TA [14,15]. The carboxyl of Glu, hydroxyl of Ser and Tyr in the derivatives can be ionized at physiologic condition, which enhances the electrostatic interaction of derivatives with DNA phosphate backbone, thus their hypochromism was increased by the interaction between charges [16]. But the different hypochromisms between 3 and 4, 1 and 2, and 5 and 6 indicated that the spatial structure, electrostatic and hydrophobic environment are important to the interaction.
Six TA derivatives were synthesized by solid-phase peptide synthesis with good yield. The preliminary interaction between derivatives and ctDNA provided valuable information in understanding the interaction mode of modified drugs with DNA, and in exploring the development of novel and highly effective peptidyl drugs.
Financial support and sponsorship:
Nil.
Conflicts of interest:
There are no conflicts of interest.
References
- Zhou XD, Tao LJ, Li L, Wu LD. Do we really need tranexamic acid in total hiparthroplasty? A meta-analysis of nineteen randomized controlled trials. Arch Orthop Trauma Sur 2013;133:1017-27.
- Karaman R, Ghareeb H, Dajani KK, Scrano L, Hallak H, Abu-Lafi S, et al.Design, synthesis and in vitrokinetic study of tranexamic acid prodrugs for the treatment of bleeding conditions. J Comput Aided Mol Des 2013;27:615-35.
- Pestourie C, Thézé B, Kuhnast B, Helleix SL, Gombert K, Dollé F, et al. PET imaging of medullary thyroid carcinoma in MEN2A transgenic mice using 6-[18F]F-L-DOPA. Eur J Nucl Med Mol Imaging 2010;37:58-66.
- Wright PM, Seiple IB, Myers AG. The evolving role of chemical synthesis in antibacterial drug discovery. AngewChemInt Edit 2014;53:8840-88.
- Yang C, Tirucherai GS, Mitra AK. Prodrug based optimal drug delivery via membrane transporter/receptor. Expert OpinBiolTher2001;1:159-75.
- Zhao DX, Sun J, Zhu QC, Lu K. Synthesis and Interaction of L-dopa-L-Tyr-L-Tyr with DNA. Chem J Chinese Universities-Chinese 2013,34:2114-9.
- Hasegawa H, Sha YL, Bang JK, Kawakami T, Akaji K, Aimoto S. Preparation of phosphopeptidethio esters by Fmoc- and Fmoc(2-F)-solid phase synthesis. LettPeptSci2001;8:277-84.
- Tang C, Ulijin RV, Saiani A. Self-assembly and gelation properties of glycine/leucineFmoc-dipeptides. EurPhys J E 2013;36:111-21.
- Monfregola L, Luca SD. Synthetic strategy for side chain mono-N-alkylation of Fmoc-amino acids promoted by molecular sieves. Amino Acids 2011;41:981-90.
- Freyer MW, Buscaglia R, Cashman D, Hyslop S, Wilson WD, Chaires JB, et al.Binding of netropsin to several DNA constructs: Evidence for at least two different 1:1 complexes formed form an-AATT-containing ds-DNA construct and a single minor groove binding ligand. BiophysChem 2007;126:186-96.
- Iwasaki Y, Kimura M, Yamada A, Mutoh Y, Tateishi M, Arii H, et al.Conformational change of ternary copper(II) complexes of cationic Schiff-bases and N-heteroaromatic amines induced by intercalative binding to DNA. InorgChemCommun 2011;14:1461-5.
- Wu JH, Wei L, Zhao M, Wang YJ, Kang GF, Peng SQ. N-[2(3-Carboxyl-9-benzyl-carboline-1-yl)ethyl-1-yl]-amino acids: correlation of spectral property with in vivo antitumor activity. Med Chem Res 2012;21:116-23.
- Guo Q, Li LZ, Dong JF, Liu HY, Xue ZC, Xu T. Synthesis, crystal structure and interactions with DNA and BSA of a oxovanadium (IV) Complex [VO(o-Van-Asn)(Phen)]·1.5 CH3OH. ActaChimSinica2012;70:1617-24.
- Cai CQ, Chen XM, Ge F. Analysis of interaction between tamoxifen and ctDNAin vitroby multi-spectroscopic methods. SpectrochimActa A 2010;76:202-6.
- Li H, Le XY, Wu JZ, Li J, Ji LN, Jiang X, et al.Studies on the interaction between copper (I) complex with phenanthroline and L-methionine ligands and DNA. ActaChim Sin 2003;61:245-50.