- *Corresponding Author:
- Selvam P
Department of Pharmaceutical Chemistry, Arulmigu Kalasalingam College of Pharmacy, Krishnankoil−636 290, India
E-mail: periyasamy_selvam@yahoo.co.in
| Date of Submission | November 10, 2010 |
| Date of Revision | June 24, 2012 |
| Date of Acceptance | June 28, 2012 |
| Indian J Pharm Sci, 2012, 74 (3): 275-278 |
Abstract
A series of novel N-substituted indophenazine derivatives were synthesised and screened for antiviral activity against a panel of human pathogenic viruses. New compounds were synthesised through modifying the N-hydrogen of indophenazine moiety with different substitution and formaldehyde by Mannich reaction. The structure of the synthetic compounds was characterised by means of infra red and nuclear magnetic resonance spectral data. The compound 10H-indolo-2-Amino pyridine [3,2-b] quinoxalines inhibits Herpes simplex virus-1 and vaccinia virus at a concentration of 12 μg/ml, and the cytotoxicy was found to be 100 μg/ml. 4-Aminobenzene sulfonamide-10H-indolo [3,2-b] quinoxalines inhibit vaccinia virus at a concentration of 12 μg/ml and cytotoxicy was found to be 100 μg/ml. The anti-HIV activities of the new compounds were also screened for in vitro antiviral activity against replication of HIV-1 (IIIB) and HIV-2 (ROD) in MT-4 cells using zidovudine (AZT) as standard. Pthalimide derivative inhibited the replication of HIV-2 (EC 50 =11.60 μg/ml and CC 50 =61.63 μg/ml) in MT-4 cells.
Keywords
HIV, Herps simplex virus, indophenazine, Mannich base, MT−4 cell, vaccinia virus
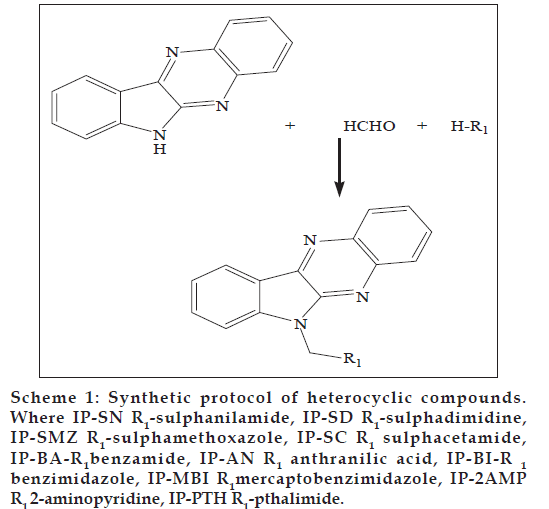
Indophenazine (10H−indolo [3,2−b] quinoxaline) is a versatile lead molecule for designing of potential bioactive agents. Indophenazine derivatives were reported to possess anticancer [1], antiviral activity against HSV [2] and inhibition of viral RNA and DNA synthesis [3], and biological activities of these compounds depended upon the functional group attached to the NH group of indophenazine moiety. 10H−indolo [3,2−b] quinoxaline is synthesised by condensing the appropriate isatin and o−phenylenediamine. We have previously reported the antiviral activity of novel heterocyclic compounds against vaccinia virus, and many of those compounds also exhibited marked cytostatic properties in lymphocytes [4−6]. Though a variety of heterocyclic compounds had been synthesised and studied for wide range of antiviral activity, the antiviral activities of indophenazine against panel of pathogenic viruses have not been extensively explored. Present work is to synthesis novel Mannich bases of indophenazine (Scheme 1) and tested for antiviral activity against HSV, vaccinia and HIV viruses. Cytotoxicity was also tested in mock−infected MT−4 and Human Foreskin cells (HEL).
Scheme 1: Synthetic protocol of heterocyclic compounds. Where IP−SN R1−sulphanilamide, IP−SD R1−sulphadimidine, IP−SMZ R1−sulphamethoxazole, IP−SC R1 sulphacetamide, IP−BA−R1benzamide, IP−AN R1 anthranilic acid, IP−BI−R 1 benzimidazole, IP−MBI R1mercaptobenzimidazole, IP−2AMP R1 2−aminopyridine, IP−PTH R1−pthalimide.
Melting points were determined in open capillary tubes on a Thomas−Hoover melting point apparatus and are uncorrected. IR spectra were recorded for KBr pellets on a (Shimadzu−800) infrared spectrophotometer, PMR spectra were determined Bruker AM, 400 MHZ with tetramethylsilane as an internal standard. The sample was dissolved in DMSO−d6 and the valves were measured in d ppm.
Synthesis of indophenazine derivatives was achieved by refluxing an equimolar (0.01 mol) mixture of formaldehyde, active hydrogen compounds (sulphanilamide, sulphadimidine, sulphamethoxazole, 2−aminopyridine, pthalamide, benzamide, nicotinamide, anthranilic acid and 2−marcapto−benzimidazole) and Indophenazine with methanol for 3 h (Scheme 1). The reaction mixture was allowed to cool overnight in refrigerator. The solid thus obtained was recrystallised from DMF. Physical data of synthesised compounds is presented in the Table 1.
| Compound | Molecular | Yield % | M.P. (º) | Rf* | Log P** |
|---|---|---|---|---|---|
| code | formula | ||||
| IP2−SN | C21H17N5O2S | 63 | 2722−280 | 0.6 | 3.72 |
| IP2−SD | C27H23N7O2S | 76 | 1902−202 | 0.4 | 5.37 |
| IP2−SMZ | C25H20N6O3S | 57 | 1752−182 | 0.53 | 4.61 |
| IP2−SC | C23H19N5O3SNa | 84 | 2252−232 | 0.8 | 3.5 |
| IP2−2AMP | C20H15N5 | 93 | 2452−253 | 0.38 | 4.14 |
| IP2−BA | C21H15N4Br | 78 | 2682−271 | 0.6 | 5.59 |
| IP2−PTH | C23H14N4O2 | 58 | 2632−270 | 0.7 | 4.36 |
| IP2−ANTH | C22H16N4O2 | 86 | 1952−205 | 0.4 | 2.78 |
| IP2−NM | C21H15N5O | 67 | 1252−132 | 0.6 | 3.9 |
| IP2−BI | C22H15N5 | 82 | 1752−195 | 0.8 | 4.11 |
| IP2−2MBI | C22H15N5S | 59 | 1632−178 | 0.7 | 3.24 |
*TLC (Chloroform:methanol 9:1) and **Log P calculated by molinspiration software.
Table 1: Physical Data Of Synthesised Compounds.
Indophenazine (10H−indolo [3,2−b] quinoxaline) (IP): IR (KBr): 3420 (NH), 1640 (C=N), 1458 (C=C); 1H NMR (DMSO−d6): 7.0-8.1 (m, 8H, Ar−H), 10.1 (b,1H, NH). 4−[(Indolo [2,3−b] quinoxalin- 6-ylmethyl)-amino]-benzenesulfonamide (IP−SN): IR (KBr): 3502 (NH), 1656 (C=N), 1451 (C=C), 1083 (>N−), 1332 (SO2), 760 (Ar−H); 1H NMR (DMSO−d6): 5.62 (s, 2H, −CH2−), 6.7-8.1 (m, 12H, Ar−H), 4.0 (b, 1H, NH), 2.0 (b, 1H, −SO2NH−). N−(4,6−Dimethyl−pyrimidin−2−yl)−4−[(indolo [2,3−b] quinoxalin−6−ylmethyl)−amino] benzenesulfonamide (IP−SD): IR (KBr): 3378 (NH), 1620 (C=N), 1424 (C=C), 1300 (SO2), 709 (Ar−H); 1H NMR (DMSO−d6): 2.35 (s, 6H, 2×CH3) 5.62 (s, 2H, −CH2−) 6.28 (d, 1H, pyrimidine), 7.0-8.1 (m, 8H, Ar−H), 10.1 (b, 1H, NH). N−Acetyl−4−[(indolo [2,3−b] quinoxalin- 6-ylmethyl)-amino]-benzene sulfonamide (IP−ISC): IR (KBr): 3383 (NH), 1663 (C=N), 1458 (C=C), 1386 (CH3), 1330 (SO2), 1093 (>N−), 762 (Ar−H); 1H NMR (DMSO−d6): 2.03 (s, 3H, −CH3) 5.62 (s, 2H, −CH2−), 6.7-7.8 (m, 12H, Ar−H), 8.2 (b, 1H, NH). 4− [(Indolo [2,3−b] quinoxalin−6−yl methyl)-amino]- N−(5-methyl-isoxazol-3−yl)-benzenesulfonamide (IP−SMZ): IR (KBr): 3383 (NH), 1663 (C=N), 1458 (C=C), 1386 (CH3), 1330 (SO2), 1093 (>N−), 762 (Ar−H); 1H NMR (DMSO−d6): 6.7-8.1 (m, 12H, Ar−H), 10.1 (b, 1H, NH), 2.03 (s, 3H, −CH3) 5.62 (s, 2H, −CH2−), 8.2 (b, 1H, SO2NH).2−Indolo [2,3−b] quinoxalin−6−ylmethyl−isoindole−1,3−dione (IP−PTH): IR (KBr): 1730(C=O), 1663 (C=N), 1458 (C=C), 1093 (>N−), 762 (Ar−H); 1H NMR (DMSO−d6): 6.2 (s, 2H, −N−CH2−N−),7.2-8.1 (m, 12H, Ar−H). Indolo [2,3−b] quinoxalin−6−ylmethyl−pyridin−2−yl−amine (IP−2AMP): IR (KBr): 3383 (NH), 1663 (C=N), 1458 (C=C), 1093 (>N−), 762 (Ar−H); 1H NMR (DMSO−d6): 4.0 (b,1H, NH), 5.6. (s, 2H, −N−CH2−N−), 6.6−8.1 (m, 12H, Ar−H). 6−Benzoimidazol−1−ylmethyl−6H−indolo [2,3−b] quinoxaline (IP−BI): IR (KBr): 1672 (C=N), 1560 (C=C), 1090 (>N−), 770 (Ar−H); 1H NMR (DMSO−d6): 6.2 (s, 2H, −N−CH2−N−), 7.1-8.0 (m,12H, Ar−H), 8.2 (b,1H, CH−Benzimidazole).
Anti−HIV Assay compounds were tested for their inhibitory effects against replication of HIV−1 (IIIB) and HIV−2 (ROD) in MT−4 cells [4]. The MT−4 cells were grown and maintained in RPMI 1640 DM Medium supplemented with 10% (v/v) heat−inactivated fetal calf serum (FCS), 2 mM glutamine, 0.1% sodium bicarbonate and 20 μg/ml gentamicin. Inhibitory effect of test compounds on HIV−1 and HIV−2 replications was monitored by inhibition of virus−induced cytopathic effect in MT−4 cells and was estimated by 3−(4,5−dimethylthiazol−2−yl)−2,5−diphenyl tetrazolium bromide (MTT) method. Briefly, 50 μl of HIV−1 and HIV−2 (100−300 CCID50) were added to a flat−bottomed microtiter tray with 50 μl of medium containing various concentrations of compounds. MT−4 cells were added at a final concentration of 6×105 cells/mL. After 5 d of incubation at 37°, the number of viable cells were determined by the MTT method. Cytotoxicity of test compounds against mock−infected MT−4 cells was also assessed by the MTT method. AntiHIV activity and cytotoxicity of compounds are presented in Table 2.
| Compound code | Strain | EC50a (µg/ml) | CC50b (µg/ml) |
|---|---|---|---|
| IP−SN | IIIB | >14.03 | 14.03 ± 2.87 |
| ROD | >14.03 | 14.03 ± 2.87 | |
| IP−SD | IIIB | >90.48 | 90.48 ± 3.48 |
| ROD | >90.48 | 90.48 ± 3.48 | |
| IP−SMZ | IIIB | >64.98 | 64.98 ± 5.92 |
| ROD | >64.98 | 64.98 ± 5.92 | |
| IP−SC | IIIB | >12.90 | 12.90 ± 0.95 |
| ROD | >12.90 | 12.90 ± 0.95 | |
| IP−2AMP | IIIB | >71.80 | 71.80 ± 8.91 |
| ROD | >71.80 | 71.80 ± 8.91 | |
| IP−BA | IIIB | >43.03 | 43.03 ± 4.58 |
| ROD | >43.03 | 43.03 ± 4.58 | |
| IP−PTH | IIIB | >61.63 | 61.63 ± 13.97 |
| ROD | 11.60 | 61.63 ± 13.97 | |
| IP | IIIB | >98.40 | 98.40 |
| ROD | >98.40 | 98.40 | |
| IP−BI | IIIB | >12.37 | 12.37 ± 0.25 |
| ROD | >12.37 | 12.37 ± 0.25 | |
| AZT (STD) | IIIB | 0.0012 ± 0.003 | 65.40 |
| ROD | 0.00016 ± 0.00027 | 65.40 |
aConcentrations required to inhibit the cytopathic effect of HIV−1(IIIB) in MT−4 cells by 50%. bConcentrations required to cause cytotoxicity to 50% of the MT−4 cells. Whereas HIV−1=(IIIB), HIV−2=(ROD). AZT−zidovudine ROD, HIV 2 strain
Table 2: Anti−hiv activity and cytotoxicity of Synthesised compounds
Antiviral activity and cytotoxicity of the synthesised compounds were determined by in vitro cell culture techniques [7]. The antiviral assays were based on inhibition of virus−induced cytopathicity in HEL (HSV−1 and HSV−2, Vaccinia) cultures. Briefly, confluent cell culture in 96−well microtiter plates were inoculated with 100 CCID50 of virus, 1 CCID50 being the virus dose required to infect 50% of the cell cultures. After 1 h virus adsorption period, residual virus was removed, and the cell cultures were incubated in the presence of varying concentrations of the test compounds. Viral cytopathicity was recorded as soon as it reached completion in the control virus−infected cell cultures exposed to the test compounds. The antiviral activity and cytotoxicity data are presented in Table 3.
| Compound | Minimum cytotoxic concentrationa (µg/ml) | EC50b (µg/ml) | ||||
|---|---|---|---|---|---|---|
| Herpes simplex virus−1 (KOS) |
Herpes simplex virus−2 (G) |
Vaccinia virus | Vesicular stomatitis virus |
Herpes simplex virus−1 TK− KOS ACVr |
||
| IP | 100 | >20 | >20 | >20 | >20 | >20 |
| IP−BI | 100 | >20 | >20 | >20 | >20 | >20 |
| IP−SC | ≥20 | >20 | >20 | >20 | >20 | >20 |
| IP−SD | ≥20 | >20 | >20 | >20 | >20 | >20 |
| IP−SMZ | 100 | >20 | >20 | >20 | >20 | >20 |
| IP−SN | 100 | 20 | >20 | 12 | >20 | 20 |
| IP−PTH | 20 | >4 | >4 | >4 | >4 | >4 |
| IP−2AMP | 100 | 12 | 20 | 12 | >20 | 20 |
| IP−BA | 100 | >20 | >20 | >20 | >20 | >20 |
| Brivudin (µM) | >250 | 0.04 | 50 | 10 | >250 | >250 |
| Ribavirin (µM) | >250 | >250 | >250 | >250 | >250 | >250 |
| Acyclovir (µM) | >250 | 2 | 2 | 7 | >250 | 2 |
| Ganciclovir (µM) | >100 | 0.06 | 0.1 | >100 | >100 | 12 |
aRequired to cause a microscopically detectable alteration of normal cell morphology. bRequired to reduce virus−induced cytopathogenicity by 50%.
Table 3:Cytotoxicity And Antiviral Activity Of Indophenazine Derivatives In Hel Cell.
We report our results from a study of replacing the NH (hydrogen) of indophenazine moiety with different types of substitutions like sulphanilamide, sulphadimidine, sulphamethoxazole, 2−aminopyridine, pthalamide, benzamide, anthranilic acid, nicotinamide, 2−marcaptobenzimidazole to form N−methyl substituted indophenazine derivatives by Mannich reaction. Synthesised compounds were screened for antiviral activity against HIV−1 and HIV−2 in MT−4 cells. Compound IP−PTH inhibits the replication of HIV−2 in MT−4 cells at a concentration of 11.60 μg/ml, and the cytotoxicy was found to be 61.63 μg/ml. All the compounds except PTH displayed cytotoxic properties in MT−4 cells. Synthesised compounds also tested for antiviral activity against HSV−1, 2 and vaccinia viruses in HEL cells. The compound 10H−indolo−2−Amino pyridine [3,2−b] quinoxalines (IP−2AMP), inhibits Herpes simplex virus−1 and vaccinia virus at a concentration of 12 μg/ml, and the cytotoxicy was found to be 100 μg/ml. incidentally the compound 4−Aminobenzene sulfonamide−10H−indolo [3,2−b] quinoxalines (IP−SN) also inhibits vaccinia virus at a concentration of 12 μg/ml, and the cytotoxicy was found to be 100 μg/ml.
Indophenazine derivatives (IP−2AMP and IP−SN) inhibited the replication of vaccinia and HSV−1 viruses below the cytotoxic concentration. Newly synthesised indophenazine derivative indolo [2,3−b] quinoxalin−6−ylmethyl−isoindole−1,3−dione (PTH) inhibited the replication of the HIV−2 in MT−4 cells. Recently, we reported the activities of certain quinazolinone derivatives with sulphanamide against biodefense viruses in cell culture [8]. The potencies of some of them exceeded those of the present indophenazine−sulphonamide series. The compounds were found to inhibit virus replication as a result of interfering with virus adsorption [9]. There is a need to discover new compounds that are inhibitory to HIV and vaccinia viruses due to the emergence of potentially pandemic virus strains and viral resistance against approved drugs.
Acknowledgement
The author is grateful to the NMR Research centre, Indian Institute of Science, Bangalore for providing the NMR facility for this research work.
References
- Smith CD, Myers CB, Zilfou JT, Smith SN, Lawrence DS. Indoloquinoxaline compounds that selectively antagonize P−glycoprotein. Oncol Res 2000;12:219−29.
- Harmenberg J, Wahren B, Bergman J, Akerfeldt S, Lundblad L. Antiherpesvirus activity and mechanism of action of indolo−(2,3−b) quinoxaline and analogs. Antimicrob Agents Chemother 1988;32:1720−4.
- Wilhelmsson LM, Kingi N, Bergman J. Interactions of antiviral indolo [2,3−b] quinoxaline derivatives with DNA. J Med Chem 2008;51:7744−50.
- Dinakaran M, Selvam P, DeClercq E, Sridhar SK. Synthesis, antiviral and cytotoxic activity of 6−bromo-2,3-disubstituted-4 (3H)-quinazolinones. Biol Pharm Bull 2003;26:1278−82.
- Selvam P, Chennama B, Murgesh N, Chandramohan M, De Clercq E. Synthesis and antiviral activity of Novel 2,3−disubstitutedquinazolin-4 (3H)-ones. Int J ChemSci 2005;2:627−31.
- Selvam P, Pandi R, Pannecouque C. Synthesis anti HIV and cytotoxicity studies of some novel N-substitutedpiperazinylfluroquinolone derivative. Indian Drugs 2007;44:626−31
- De Clercq E. Antiviral and antimetabolic activities of neplanocins. Antimicrob Agents Chemother 1985;28:84−9.
- Selvam P, Vijayalakshimi P, Smee DF, Gowen BB, Julander JG, Day CW, et al. Novel 3−sulphonamido−quinazolin−4 (3H)−one derivatives: Microwave−assisted synthesis and evaluation of antiviral activities against respiratory and biodefense viruses. AntivirChemChemother 2007;18:301−5.
- Selvam P, Murugesh N, Chandramohan M, Sidwell RW, Wandersee MK, Smee DF. Anti−influenza virus activities of 4-[(1,2-dihydro-2-oxo-3H-indol- 3-ylidene) amino]-N-(4,6−dimethyl-2-pyrimidin-2-yl) benzenesulphonamide and its derivatives. AntivirChemChemother 2006;17:269−74.