- *Corresponding Author:
- Aalisha
Department of Veterinary Pharmacology and Toxicology, College of Veterinary Science and Animal Husbandry, Dau Shri Vasudev Chandrakar Kamdhenu Vishwavidyalaya (DSVCKV), Durg, Chhattisgarh 491001, India
E-mail: iamaalisha0412@gmail.com
| Date of Received | 16 June 2025 |
| Date of Revision | 14 August 2025 |
| Date of Accepted | 21 October 2025 |
| Indian J Pharm Sci 2025;87(5):209-219 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Diabetes mellitus, affecting approximately 25 % of the global population, remains a significant health challenge due to limited effective treatments. Nanotechnology, particularly green-synthesized silver nanoparticles, offers promising potential for innovative drug delivery systems. Allium sativum (Garlic), known for its antidiabetic properties, can be utilized in combination with nanotechnology to enhance therapeutic efficacy. This study aimed to evaluate the antidiabetic activity of green-synthesized silver nanoparticles prepared using aqueous garlic extract according to standard procedures and their effect was assessed on relevant enzymes associated with diabetes. For this thirty-six adult Wistar albino rats were divided into six groups each having six animals. The induction of diabetes in rats was by a single intraperitoneal injection of alloxan (150 mg/kg body weight). Rats were grouped as follows: Group I (NC): Normal control treated with distilled water (0.1 ml/kg b.wt.). Group II (DC): Diabetic control without any treatment. Group III (Standard): Treated with Glibenclamide (500 µg/kg b.wt.). Group IV (Garlic Extract): Treated with alcoholic garlic extract (400 mg/kg b.wt.). Group V (Silver nanoparticle): Treated with garlic antidiabetic activity of green-synthesized silver nanoparticles (80 mg/kg b.wt.). Group VI (Garlic extract+standard): Treated with a combination of Glibenclamide (500 µg/kg b.wt.) and garlic extract (400 mg/kg b.wt.). Treatment was initiated after 72 h of diabetes induction and continued for 15 d. Characterization of antidiabetic activity of green-synthesized silver nanoparticles was performed using ultra violet-visible spectrometry, Zeta potential analysis, and scanning electron microscopy. The results revealed that the synthesized antidiabetic activity of green-synthesized silver nanoparticles exhibited spherical to irregular shapes with an average size of 45 nm. The blood glucose levels were significantly lower (p<0.05) in rats administered Garlic antidiabetic activity of green-synthesized silver nanoparticles when compared with the diabetic control group. Furthermore, administration of Garlic extracts and Garlic antidiabetic activity of green-synthesized silver nanoparticles attenuated oxidative stress by decreasing malondialdehyde levels, enhancing reduced glutathione activities and improved serum protein levels, reinstated the lipid profiles and renal parameters to nearly normal levels in diabetic rats. The results reveal that Garlic-antidiabetic activity of green-synthesized silver nanoparticles exert their antidiabetic effects through the modulation of glycemic and atherogenic indices as well as the mitigation of free-radical-mediated damages. The present findings revealed that Garlic silver nanoparticles significantly improved the alloxan-induced diabetic changes in various treated rats and might be used in the treatment/ management of diabetes.
Keywords
Silver nanoparticle, characterization, zeta potential, SEM, ultra violet-visible spectrometry, glibenclamide, antidiabetic, Allium sativum
Diabetes mellitus is a severe, prolonged, and chronic metabolic disorder that results in hyperglycemia due to reduced insulin production (type I) or inefficient insulin utilization (type 2)[1]. Diabetes is the second largest disease, affecting 25 % of the global population. In diabetic patients, the insulin will not be efficiently acted on the glucose metabolism which leads to the synthesis of a large amount of glucose in the liver by the action of glucagon[2]. Most of the synthetic medicines available for the treatment of diabetes have side effects and cannot be used during pregnancy[3]. The usage of medicinal plants for the treatment of diabetes begins from the time immortal. But they failed to cure the disease completely[4]. Although there are numerous medicines for the control and maintenance of normal blood sugar levels, there is no single medicine for the complete remedy of this disease. So the identification of an antidiabetic agent that has to stimulate insulin activity as well as to reduce/ inhibit glucagon activity is a major challenge for the modern scientific world.
The World Health Organization reported that more than 80 % of the population of the world uses traditional medicine as a drug for its major healthcare requirements. There is a long history of the usage of herbal drugs in Asia that shows interactions with the environment. For the treatment of infectious as well as chronic diseases, traditional medicinal plants have been used[5]. The search for effective therapeutic agents derived from natural sources has gained considerable momentum in the field of medical research. Among these sources, Garlic Allium sativum (A. sativum) have long been recognized for their diverse pharmacological properties and potential health benefits. In particular, its traditional use in the management of diabetes has attracted scientific interest, leading to extensive investigations aimed at unravelling the underlying mechanisms.
In recent times, the application of nanotechnology has opened up new avenues for drug discovery and delivery, enhancing the therapeutic potential of natural compounds. Among the various nanoparticles used in biomedical research, silver nanoparticles have gained considerable attention due to their unique physicochemical properties and wide ranging applications. Their utilization as carriers for herbal extracts has shown promising results, including improved bioavailability and targeted drug delivery. Antidiabetic Activity of Green-Synthesized Silver Nanoparticles (AgNPs) are being used as antifungal, antibacterial[6], anti-inflammatory, and antioxidant agents[7]. Green production of AgNPs using different extracts of florae including Bauhinia variegate[8], Anthemis atropatana[9], Caesalpinia pulcherrima[10], Cassia fistula[11], Euphrasia officinalis[12], Glycyrrhiza uralensis[13], Indigofera tinctoria[14], Bergenia ciliate[15], and Ipomoea batatas[16] have been successfully reported.
Garlic (A. sativum), a traditional medicinal plant with a huge amount of medicinal properties, showed better hypoglycaemic activities in rats and humans[17,18]. The antidiabetic activity of A. sativum was well documented in terms of reducing insulin resistance and blood sugar level[19]. The sulphur-containing compounds (alliin, allicin, ajoene, diallyl disulfide, diallyl trisulfide, diallyl sulfide, S-allyl cysteine, and allyl mercaptan) from A. sativum is responsible for the pungent smell and have anti-diabetic activity[20]. The phyto compounds such as sitagliptin, vildagliptin, and alogliptin from A. sativum were used to treat diabetes clinically[21]. The AgNPs were also synthesized from A. sativum which showed different bioactive properties like antimicrobial, antiinflammatory, antioxidant, anticoagulant, anticancer and antidiabetic activities[22-25].
This research work provides a comprehensive overview of the pharmacological screening conducted on garlic silver nanoparticles, with a specific focus on its antidiabetic activity. By harnessing the combined benefits of nanotechnology and these medicinal plants, researchers hope to develop innovative therapeutic strategies in diabetes management. Ultimately, the findings from this research will contribute to our understanding of the antidiabetic potential of garlic silver nanoparticles. The outcomes may pave the way for the development of novel Nano therapeutic interventions with enhanced efficacy and reduced side effects, ultimately benefiting individuals suffering from diabetes.
Materials and Methods
The present investigations consisted of the screening of Garlic (A. sativum) for pharmacological actions in laboratory animals.
Preparation of garlic extract:
The Garlic powder was subjected to continuous extraction with hot methanol by using Soxhlet’s apparatus. The extract so obtained was weighed and transferred into an air tight screw cap vial and stored in refrigerator for use, whenever required. This hot methanolic Garlic extract was referred hereafter as “GE”.
Synthesis of garlic silver nanoparticles:
In green synthesis of AgNPs, 1 mm of aqueous solution of silver nitrate was prepared freshly. 25 ml aqueous Garlic extract and 100 ml silver nitrate solution was mixed in a conical flask (1:4) and heated in sand bath at 60° for 30 min. AgNP precipitate was centrifuged at 6000 rpm for 15 min, pellets were redisposed in ethanol and washed thrice. This AgNP pellet were dried in oven to obtain powder[26].
Characterization of silver nanoparticles:
Characterization of Garlic silver nanoparticle was done by Ultra Violet-Visible (UV-Vis) spectrometry, Scanning Electron Microscopy (SEM) analysis and zeta potential analysis.
UV-Vis spectrometry: Absorbance was measured by UV-VIS spectra on a wave length from 300 nm to 800 nm (UV-VIS spectrophotometer 117, Systronics). Spectrum was taken and synthesis of silver nanoparticles was confirmed by peak observation.
SEM analysis: The size and morphology of powdered AgNps were analysed at different magnifications using SEM equipped with digital imaging that works in low and high vaccum mode (MNCF, CeNSE, IISc, Bengaluru, India).
Zeta particle size analysis: Zeta analysis was used to determine the zeta size and zeta potential of Garlic nanoparticles. The particle size distribution width was determined by the average hydrodynamic diameter measurement, the peak values in the hydrodynamic diameter distribution, and the Polydispersity Index (PDI) of the particles (MNCF, CeNSE, IISc, Bengaluru, India).
Experimental animals:
Albino rats obtained from Lab Animal House, College of veterinary science and AH, Anjora were used for the study. The animals were housed in poly propylene rat animal cages and maintained on balanced lab animal feed and clean drinking water in climatized Lab Animal House for a period of 30 d with prior approval by the Institutional Animal Ethical Committee (IAEC), College of Veterinary Science & A.H., Anjora.
Albino rats in 8 groups, 2 groups for acute toxicity (n=5), and 6 groups (n=6) for antidiabetic study were used.
Acute toxicity study:
Limit test was performed in 5 female rats to evaluate the acute toxicity of Garlic extract and Garlic AgNPs. One female rat was orally administered with maximum dose of Garlic extract i.e. 2000 mg/kg. It was observed for 14 d for any mortality, signs and symptoms of acute toxicity. Then the same dose was given sequentially to another four female rats so as to test a total five animals. Similarly the same process was applied for acute toxicity test of Garlic AgNPs.
Experimental design for evaluation of antidiabetic activity:
Experimental animals were divided into six groups containing six animals in each group. The animals of Group 1 were given normal saline intraperitoneally and were kept as normal control. Diabetes was induced in rats of group 2,3,4,5, and 6. The rats of group 2 were not treated with any compound and kept as diabetic control. The rats of group 3,4,5 and 6 were treated with oral administration of standard drug Glibenclamide @500 ug/kg, Garlic extract @400 mg/kg, Garlic silver nanoparticle @80mg/ kg and combination of Garlic extract @400 mg/ kg+Standard drug Glbenclamide @500 μg/kg respectively for 15 d (Table 1).
| Group | Treatments | No. of days of treatment | No. of animals |
|---|---|---|---|
| 1 | Normal control, given distilled water (1 ml/kg b.wt., orally) | 15 | 6 |
| 2 | Diabetic control (Alloxan @150 mg/kg b.wt.) single dose I/P | - | 6 |
| 3 | Diabetic rats treated with Garlic NP @ 80 mg/kg b.wt., orally, daily. | 15 | 6 |
| 4 | Diabetic rats treated with alcoholic extract of garlic @400 mg/kg b.wt., orally, daily . | 15 | 6 |
| 5 | Diabetic rats treated with Glibenclamide (Standard reference drug @500 ug/kg, orally), daily | 15 | 6 |
| 6 | Diabetic rats treated with combination of Glibenclamide @500 ug/ kg+Garlic extract @ 400 mg/kg b.wt.orally,. | 15 | 6 |
Table 1: Experimental Design for Evaluation of Antidiabetic Activity
Induction of diabetes in rats and blood glucose determination:
The 30 rats were fasted overnight prior to injection of alloxan dissolved in iced cold normal saline at a dose of 150 mg/kg body weight and the routes of administration were intraperitoneal injection [27]. After 1 h of alloxan administration, the rats were fed 5 % dextrose solution in a feeding bottle for a day to overcome the early hypoglycaemic phase. The rats were kept under observation and after 2 d the blood glucose was confirmed using one touch glucometer.
The anti-diabetic activity of garlic silver nanoparticle were evaluated on the basis of following parameters:
Body weight of the animal: Animals in all groups were weighed using digital weighing machine on 0 d (Pre-Treatment), d 7 and d 15 (post treatment).
Biochemical analysis: Blood glucose was tested by Accuchek one touch glucometer by taking blood from tail vein on d 0, d 07 and d 15. Other biochemical parameters viz. total protein, cholesterol, triglycerides, High-Density Lipoprotein (HDL), Low-Density Lipoprotein (LDL), Blood Urea Nitrogen (BUN), creatinine and uric acid were estimated from serum samples. On d 15 of experiment, blood samples were collected from all the animals for biochemical evaluation, blood samples were collected in centrifuge tubes without anticoagulant and allowed to clot at room temperature. Serum was harvested by centrifugation at 3000 rpm for 10 min and stored at -4° until biochemical analysis.
Statistical analysis:
Statistical analysis was done using Completely Randomized Design (CRD), one-way classification as per procedure given by Snedecor and Cochran (1994) [28]. Duncan’s Multiple Range Test was employed for the significant differences amongst the different treatments.
Results and Discussion
UV-Vis spectroscopy is the effective and simple technique and used primarily for characterization and determination of the stability and the optical properties. The formation of silver nanoparticles was primarily monitored by using the UV-Vis Spectrophotometer at a wavelength range of 300- 800 nm. A single, strong and broad Surface Plasmon Resonance (SPR) peak in UV-visible spectrum of the green synthesized silver nanoparticle using the Garlic (A. sativum) extract was observed at 450 nm (fig. 1). These results are corroborated with Megiel[29], who has also reported SPR peak located within 410-460 nm region and also observed the relation between particle size and the plasmon peak, which shift to longer wavelengths as the particles became larger. Elamawi et al.[30] reported peak at wavelength of UV spectra at 383 nm with particle size at 1-25 nm at 28°. Similar results is also reported by Veerasamy et al.[31], who has reported that on addition of different concentration (1-5 ml) of extracts to aqueous silver nitrate solution keeping its concentration 10 ml (1 ml) constant, the colour of the solution changed from faint light to yellowish brown and finally to colloidal brown indicating formation of silver nanoparticles.
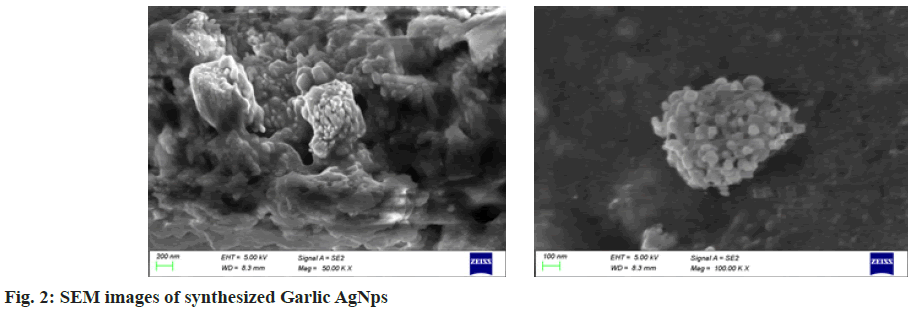
The results of SEM confirmed the presence of nanoparticles in the reaction mixture. The synthesized nanoparticles were spherical in shape and were uniformly distributed (fig. 2). The spherical shape of the synthesized AgNPs was observed, and they were more aggregated. Their average size was found to be 45.5 nm. Our result corresponded with the result of El Refai et al.[32], his study revealed the presence of monodispersed metal nanoparticles and silver nanoparticles of ginger have particle size 10.10-18.33 nm.
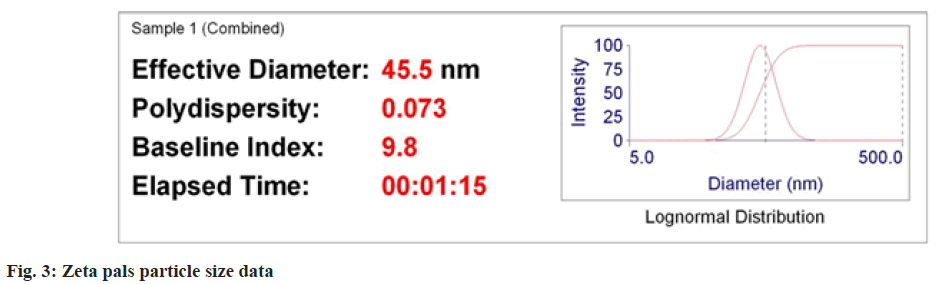
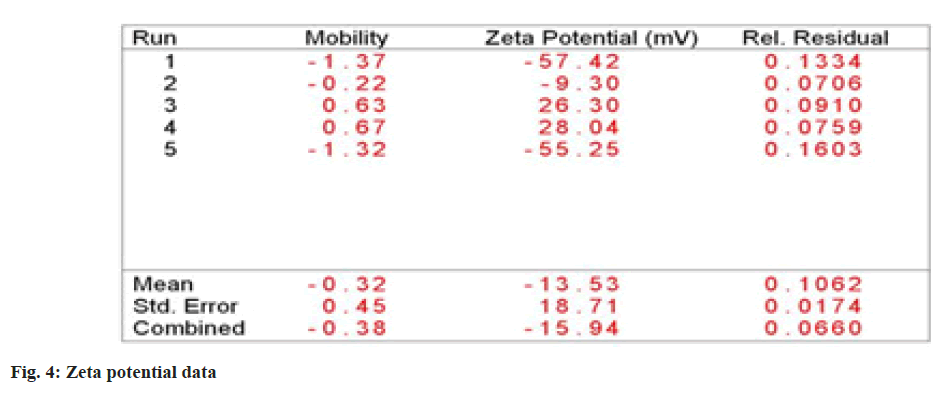
The results of zeta analysis showed that the average size of nanoparticle formed was 45 nm (fig. 3 and fig. 4). Zeta potential analysis (ζ) demonstrated that garlic silver nanoparticle had negative charge of -13.53 mV, indicating that they are sufficiently charged to maintain stability in solution over long periods of time. The results were in corroboration with White et al.[33], who also found that the Garlic extract nanoparticles have negative charges indicating its stability. Similar results were found by Ali et al.[34], having garlic silver nanoparticles with negative charge indicating stability.
Acute toxicity studies:
This study showed no mortality up to the dose of 2000 mg/kg body weight. So, the extract as well as AgNP is safe for long term administration. Our results are in agreement with Nakagawa et al.[35], who found that the garlic nanoparticles have LD50 of garlic extract is very higher, hence it is safe for oral administration up to dose rate of 200 mg/kg. Also Galliet and Rouanet et al.[36] found that the toxic effects and the interactions of AgNps with biological systems is necessary to handle these nanoparticles and their use. They usually generate reactive oxygen species resulting in pro antiinflammatory reactions. Thus finding it safe for oral administration.
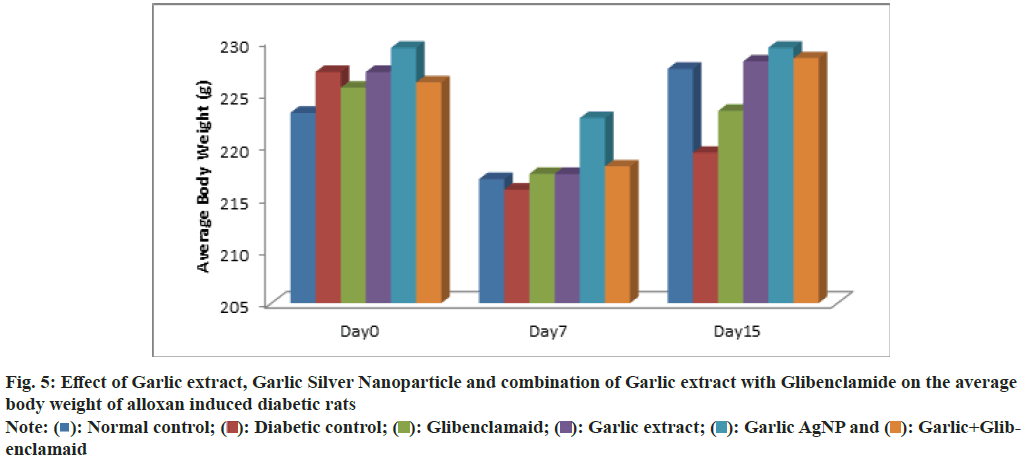
The average body weight of albino rats treated with garlic AgNPs, garlic extract, and garlic extract+glibenclamide was recorded over 15 d (Table 2 and fig. 5). On d 0, the body weights for Group 1 (Normal Control), Group 2 (Diabetic Control), Group 3 (Standard), Group 4 (Garlic Extract), Group 5 (Garlic AgNPs), and Group 6 (Garlic extract+Glibenclamide) were 223.1±13.746 g, 227±18.740 g, 225.5±12.177 g, 227±12.680 g, 229.3±10.838 g, and 226±13.755 g, respectively, with no significant differences (p<0.05), indicating proper randomization. After 7 d, weights were 211.6±11.651 g, 215.8±23.163 g, 217.3±13.063 g, 217.3±17.247 g, 222.6±11.021 g, and 218±13.446 g, respectively, again with no significant differences (p<0.05). By d 15, the weights for Groups 1-6 were 227.3±13.735 g, 219.3±25.982 g, 223.3±13.633 g, 228±14.953 g, 229.3±10.838 g, and 228.3±13.764 g. Groups 3, 4, 5, and 6 had significantly higher weights compared to Group 2 (Diabetic Control), but no significant difference was noted between Groups 1 and 2.
Fig. 5: Effect of Garlic extract, Garlic Silver Nanoparticle and combination of Garlic extract with Glibenclamide on the average body weight of alloxan induced diabetic rats
Note: ( ): Normal control; (
): Normal control; ( ): Diabetic control; (
): Diabetic control; ( ): Glibenclamaid; (
): Glibenclamaid; ( ): Garlic extract; (
): Garlic extract; ( ): Garlic AgNP and (
): Garlic AgNP and ( ): Garlic+Glibenclamaid
): Garlic+Glibenclamaid
Table 2: Effect of Supplementation of Garlic Agnpsand Combination of Garlic Agnps+Glibenclamide on Average Body Weight of Alloxan Induced Diabetic Rats
| Group | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|
| Treatment | Normal control | Diabetic control | Standard glibenclamide | Garlic extract | Garlic nanoparticle | Garlic Extract+Glibenclamide | Levelof Significance |
| Average body weight (g)/rat 0 d |
223.1±13.746 | 227±18.740 | 225.5±12.177 | 227±12.680 | 229.3±10.838 | 226±13.755 | NS |
| 7 d | 216.8±11.651 | 215.8±23.163 | 217.3±13.063 | 217.3±17.247 | 222.6±11.021 | 218±13.446 | NS |
| 15 d | 227.3±13.735ab | 219.3±25.982b | 223.3±13.633a | 228±14.953a | 229.3±10.838a | 228.3±13.764a | * |
Table 2: Effect of Supplementation of Garlic Agnpsand Combination of Garlic Agnps+Glibenclamide on Average Body Weight of Alloxan Induced Diabetic Rats
The result of this study showed increase in the mean body weight of animals with significant percentage weight changes in the groups treated with garlic nanoparticle and garlic extract, as well as the combination of Garlic extract+Glibenclamide, exhibited significantly higher average body weights compared to the diabetic control group (p<0.05). However, the normal control and standard glibenclamide groups did not show significant differences in body weight compared to the diabetic control group (p>0.05) indicating that the different phytochemicals in the extract specially phenols are responsible for growth promotion due to its antioxidant properties this observation agrees with the reports of Mahmood et al.[37] and Tanko et al.[38] which recorded an increase in the mean body weight of rat-fed plant extract.
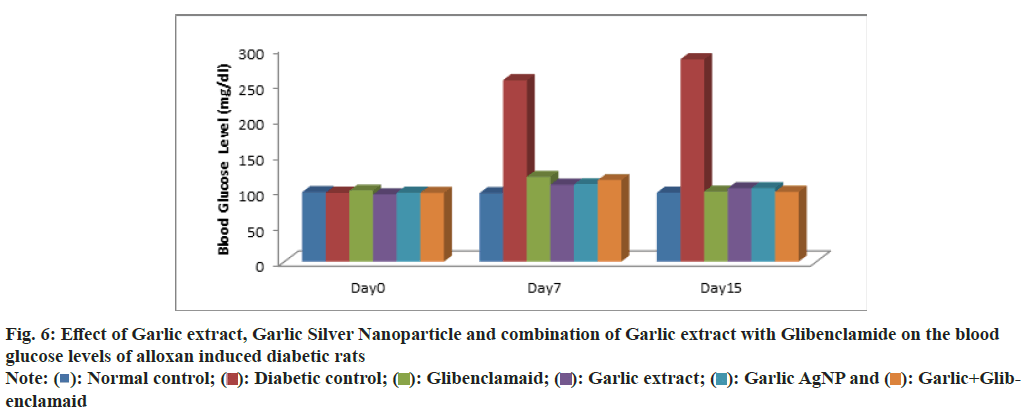
The average blood glucose levels of the experimental groups were measured at 0, 7, and 15 d. On d 0, the blood glucose levels for Groups 1 (normal control), 2 (diabetic control), 3 (standard), 4 (garlic extract), 5 (Garlic AgNPs), and 6 (Garlic extract+Glibenclamide) were 97.5±1.048, 96.6±1.211, 96.6±6.1751, 94.3±3.386, 99.6±1.751, and 96.5±1.870 mg/dl, respectively, with no significant differences (p>0.05), indicating proper randomization. On d 7, blood glucose levels were 96±1.788, 254.5±8.336, 108.5±6.565, 108±5.537, 118.8±7.167, and 114.3±7.44 mg/dl for Groups 1-6, respectively. Group 2 (Diabetic Control) showed significantly higher glucose levels compared to treatment groups (p<0.05). Among treatments, no significant differences were observed between Groups 5 and 6 or between Groups 3 and 4. At the end of the experiment (d 15), glucose levels for Groups 1-6 were 96.6±0.816, 283.8±8.863, 103±4.000, 103±4.226, 98.3±4.033, and 98±2.828 mg/dl, respectively. Group 2 had a 3-fold increase in glucose (p<0.05) compared to controls. Treatments (Groups 3-6) significantly reduced glucose levels, with the lowest levels observed in Group 6 (Garlic extract+Glibenclamide), comparable to the control. No significant differences were found among Groups 3, 4, 5, and 6 (Table 3 and fig. 6).
Table 3: Average body weight (g)/rat of experimental groups
| Levelof Treatment | Group 1 Normal control |
Group 2 Diabetic control |
Group 3 Standard glibenclamide |
Group 4 Garlic extract |
Group 5 Garlic nanoparticle |
Group 6 Garlic Extract+Glibenclamide |
Significance |
|---|---|---|---|---|---|---|---|
| 0 d | 97.5±1.048ab | 96.6±1.211ab | 96.6±1.751ab | 94.3±3.386b | 99.6±6.772a | 96.5±1.870ab | NS |
| 7 d | 96±1.788d | 254.5±8.336a | 108.5±6.565c | 108±5.537c | 118.8±7.167b | 114.3±7.44bc | * |
| 15 d | 96.6±0.816c | 283.8±8.863a | 103±4.000b | 103±4.226b | 98.3±4.033bc | 98±2.828bc | * |
Table 3: Effect of Supplementation of Garlic Agnps and Combination of Garlic Agnps+Glibenclamide on Average Blood Glucose Levels of Alloxan Induced Diabetic Rats
Fig. 6: Effect of Garlic extract, Garlic Silver Nanoparticle and combination of Garlic extract with Glibenclamide on the blood glucose levels of alloxan induced diabetic rats
Note: ( ): Normal control; (
): Normal control; ( ): Diabetic control; (
): Diabetic control; ( ): Glibenclamaid; (
): Glibenclamaid; ( ): Garlic extract; (
): Garlic extract; ( ): Garlic AgNP and (
): Garlic AgNP and ( ): Garlic+Glibenclamaid
): Garlic+Glibenclamaid
The significant elevation in glucose levels in diabetic rats is attributed to the cytotoxic effects of alloxan, which damages pancreatic beta cells through superoxide radical production. Garlic extract and Garlic AgNPs showed significant antidiabetic effects, likely by enhancing plasma insulin activity, promoting glucose uptake, inhibiting hepatic gluconeogenesis, or stimulating beta-cell regeneration. These findings align with Jini et al.[39], who reported that Garlic AgNPs increased glucose utilization, inhibited α-amylase and α-glucosidase, and reduced hepatic glucose production, without directly stimulating insulin secretion. Molecular docking studies further confirmed the interaction of AgNPs with key enzymes and insulin-related residues, supporting their antidiabetic potential.
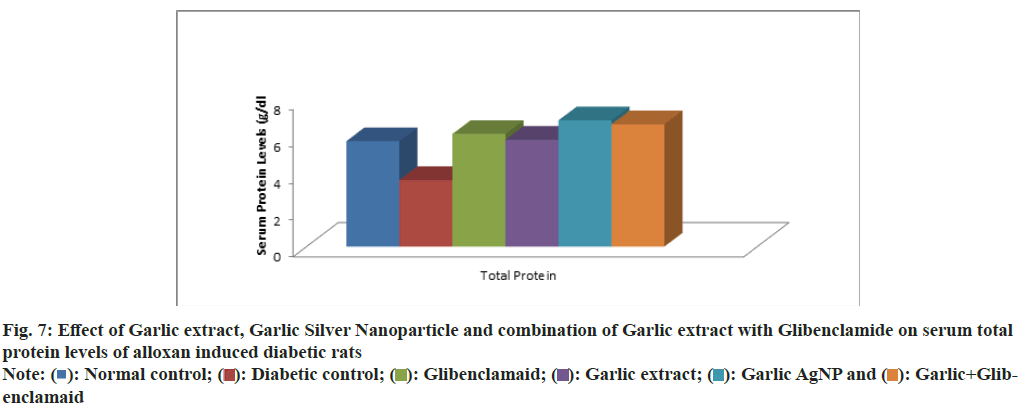
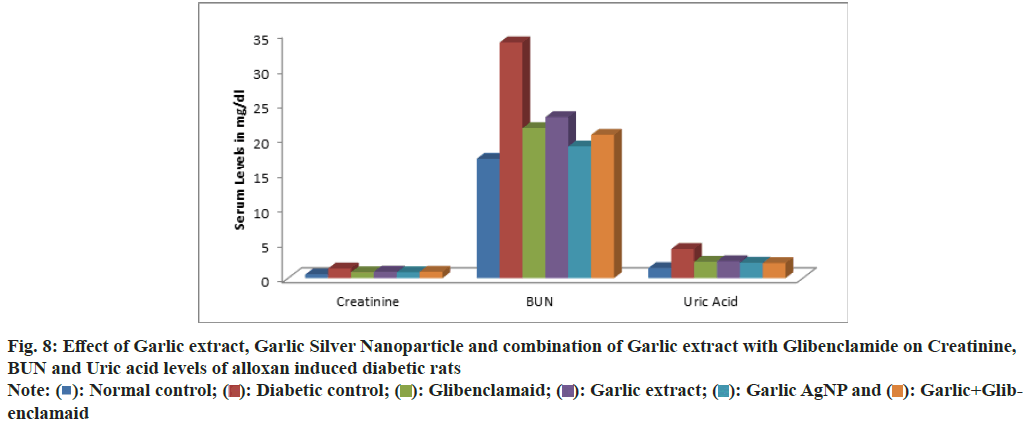
Serum levels of total protein, creatinine, BUN, uric acid and lipid profile across experimental groups are summarized in Table 4. The total protein levels in Group 1: 5.721±0.161, Group 2: 3.611±0.447, Group 3: 6.851±0.274, Group 4: 5.803±0.861, Group 5: 6.113±0.650, Group 6: 6.648±0.261 (fig. 7). The creatinine levels are: Group 1: 0.533±0.108, Group 2: 1.391±0.307, Group 3: 0.851±0.046, Group 4: 0.941±0.045, Group 5: 0.850±0.066, Group 6: 0.906±0.043. BUN levels are: Group 1: 17.073±2.157, Group 2: 33.711±3.27, Group 3: 21.465±1.968, Group 4: 23.033±2.089, Group 5: 18.833±1.732, Group 6: 20.490±1.581. The Uric Acid levels are: Group 1: 1.451±0.273, Group 2: 4.173±0.251, Group 3: 2.331±0.323, Group 4: 2.428±0.342, Group 5: 2.211±0.319, Group 6: 2.153±0.219 (fig. 8).
| Group | 1 | 2 | 3 | 4 | 5 | 6 | Level of significance |
|---|---|---|---|---|---|---|---|
| Treatment | Normal control | Diabetic control | Standard glibenclamide | Garlic extract | Garlic nanoparticle | Garlic extract+glibenclamiode | |
| Total protein (g/dl) | 5.721±0.161c | 3.611±0.447d | 6.113±0.650bc | 5.803±0.861c | 6.851±0.274a | 6.648±0.261ab | * |
| Total cholesterol (mg/dl) | 96.871±14.276c | 169.845±12.867a | 121.376±11.007b | 120.431±16.106b | 87.833±8.717c | 100.738±12.801c | * |
| Triglycerides (mg/dl) | 126.2±14.549c | 206.516±34.120a | 139.093±15.607bc | 150.92±7.831b | 126.003±2.073c | 154.1083±11.872b | * |
| HDL (mg/dl) | 39.17±7.776bc | 34.14±2.783d | 42.631±3.393b | 40.115±4.902bc | 49.673±5.144a | 44.786±4.150ab | * |
| LDL (mg/dl) | 61.473±2.410d | 91.255±6.009a | 77.008±4.566b | 76.078±3.342b | 71.041±2.621c | 69.866±2.835c | * |
| Creatinine (mg/dl) | 0.533±0.108c | 1.391±0.307a | 0.851±0.046b | 0.941±0.045b | 0.850±0.066b | 0.906±0.043b | * |
| BUN (md/dl) | 17.073±2.157d | 33.711±3.27a | 21.465±1.968bc | 23.033±2.089b | 18.833±1.732cd | 20.490±1.581bc | * |
| Uric acid (mg/dl) | 1.451±0.273c | 4.173±0.251a | 2.331±0.323b | 2.428±0.342b | 2.211±0.319b | 2.153±0.219b | * |
Table 4: Effect of Supplementation of Garlic Agnpsand Combination of Garlic Agnps+Glibenclamide on Biochemical Parameters of Alloxan Induced Diabetic Rats
Fig. 7: Effect of Garlic extract, Garlic Silver Nanoparticle and combination of Garlic extract with Glibenclamide on serum total protein levels of alloxan induced diabetic rats
Note: ( ): Normal control; (
): Normal control; ( ): Diabetic control; (
): Diabetic control; ( ): Glibenclamaid; (
): Glibenclamaid; ( ): Garlic extract; (
): Garlic extract; ( ): Garlic AgNP and (
): Garlic AgNP and ( ): Garlic+Glibenclamaid
): Garlic+Glibenclamaid
Fig. 8: Effect of Garlic extract, Garlic Silver Nanoparticle and combination of Garlic extract with Glibenclamide on Creatinine, BUN and Uric acid levels of alloxan induced diabetic rats
Note: ( ): Normal control; (
): Normal control; ( ): Diabetic control; (
): Diabetic control; ( ): Glibenclamaid; (
): Glibenclamaid; ( ): Garlic extract; (
): Garlic extract; ( ): Garlic AgNP and (
): Garlic AgNP and ( ): Garlic+Glibenclamaid
): Garlic+Glibenclamaid
Diabetic rats (Group 2) exhibited decreased total protein and elevated BUN, creatinine, and uric acid levels, indicating metabolic and renal dysfunction associated with hyperglycaemia. Treatment with garlic AgNPs (Group 5) and garlic extract+glibenclamide (Group 6) significantly restored these parameters, corroborating findings by Anwar et al.[40] on the Reno protective effects of fenugreek seed extract in diabetic rats. Elevated BUN, uric acid, and creatinine levels highlight renal dysfunction in diabetes.
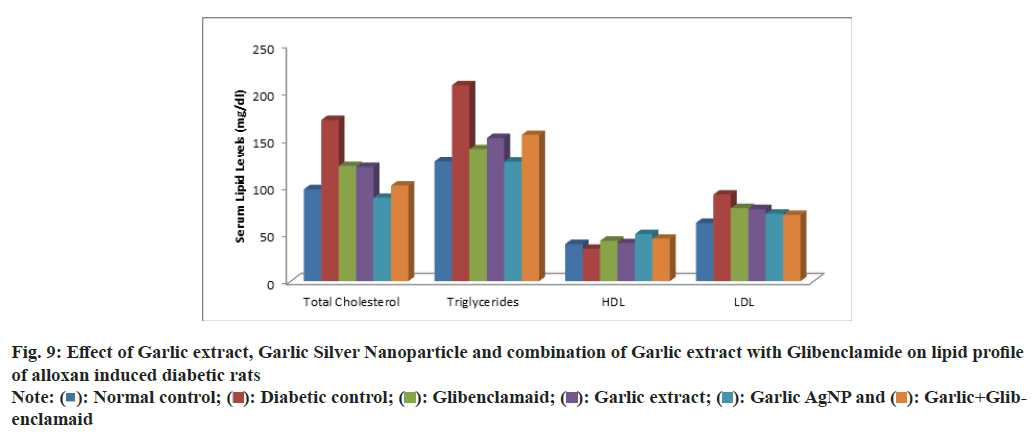
The total cholesterol levels (mg/dl) in Group 1: 96.87±14.28, Group 2: 169.85±12.87, Group 3: 87.83±8.72, Group 4: 120.43±16.11, Group 5: 121.38±11.01 and Group 6: 100.74±12.80. Triglyceride levels (mg/dl) were found to be as follows Group 1: 97.89±10.67, Group 2: 181.27±12.45, Group 3: 91.71±8.92, Group 4: 123.35±14.24, Group 5: 117.52±10.42, Group 6: 103.28±13.19. HDL levels (mg/dl) are as follows Group 1: 39.17±7.78, Group 2: 34.14±2.78, Group 3: 42.63±3.39, Group 4: 40.12±4.90, Group 5: 49.67±5.14, Group 6: 44.79±4.15. LDL levels (mg/dl) were as follows Group 1: 61.47±2.41, Group 2: 91.26±6.01, Group 3: 77.01±4.57, Group 4: 76.08±3.34, Group 5: 71.04±2.62, Group 6: 69.87±2.83. Diabetic rats (Group 2) exhibited hypercholesterolemia, hypertriglyceridemia, reduced HDL, and elevated LDL, indicating dyslipidemia. Treatment with garlic extract, garlic AgNPs, and their combination (Groups 3, 4, 5, and 6) significantly improved lipid profiles. Notably, HDL levels increased, and LDL, cholesterol, and triglycerides were reduced, comparable to normal levels in Groups 5 and 6 (fig. 9).
Fig. 9: Effect of Garlic extract, Garlic Silver Nanoparticle and combination of Garlic extract with Glibenclamide on lipid profile of alloxan induced diabetic rats
Note: ( ): Normal control; (
): Normal control; ( ): Diabetic control; (
): Diabetic control; ( ): Glibenclamaid; (
): Glibenclamaid; ( ): Garlic extract; (
): Garlic extract; ( ): Garlic AgNP and (
): Garlic AgNP and ( ): Garlic+Glibenclamaid
): Garlic+Glibenclamaid
These results align with Keshetty et al.[41] and Manam & Subbaiah et al.[42], who observed hypolipidemic effects of garlic and AgNPs, including increased HDL and decreased TC, TG, and LDL. The lipidlowering effects may result from inhibition of lipogenic enzymes, enhanced cholesterol excretion, and suppression of LDL oxidation. Increased HDL facilitates cholesterol removal, potentially slowing atherosclerosis as reported by Nofer et al.[43] and Methew& Biju et al.[44].
Conflict of interests:
The authors declared no conflict of interests.
References
- Prabhakar PK, Doble M. Effect of natural products on commercial oral antidiabetic drugs in enhancing 2-deoxyglucose uptake by 3T3-L1 adipocytes. Ther Adv Endocrinol Metab 2011;2(3):103-14.
[Crossref] [Google scholar] [PubMed]
- Rizza RA. Pathogenesis of fasting and postprandial hyperglycemia in type 2 diabetes: implications for therapy. Diabetes 2010;59(11):2697-707.
[Crossref] [Google Scholar] [PubMed]
- Fullerton B, Siebenhofer A, Jeitler K, Horvath K, Semlitsch T, Berghold A, et al. Short?acting insulin analogues vs. regular human insulin for adult, non?pregnant persons with type 2 diabetes mellitus. Cochrane Database Syst Rev 1996;2018(12):CD013228.
[Crossref] [Google Scholar] [PubMed]
- Salehi B, Ata AV, Anil Kumar N, Sharopov F, Ramirez-Alarcon K, Ruiz-Ortega A, et al. Antidiabetic potential of medicinal plants and their active components. Biomolecules 2019;9(10):551.
[Crossref] [Google Scholar] [PubMed]
- Chashoo IA, Dinesh Kumar DK, Bhat ZA, Khan NA, Vijender Kumar VK, Nowshehri JA. Antimicrobial studies of Sambucus wightiana Wall. ex. Wight & Arn. J Pharm Res 2012;5:2467-8.
- Oh KH, Soshnikova V, Markus J, Kim YJ, Lee SC, Singh P, et al. Biosynthesized gold and silver nanoparticles by aqueous fruit extract of Chaenomeles sinensis and screening of their biomedical activities. Artif Cells Nanomed Biotechnol 2018;46(3):599-606.
[Crossref] [Google Scholar] [PubMed]
- Wong KK, Cheung SO, Huang L, Niu J, Tao C, Ho CM, et al. Further evidence of the anti?inflammatory effects of silver nanoparticles. Chem Med Chem 2009;4(7):1129-35.
[Crossref] [Google Scholar] [PubMed]
- Johnson P, Krishnan V, Loganathan C, Govindhan K, Raji V, Sakayanathan P, et al. Rapid biosynthesis of Bauhinia variegata flower extract-mediated silver nanoparticles: An effective antioxidant scavenger and α-amylase inhibitor. Artif Cell Nanomed Biotechnol 2018;46(7):1488-94.
[Crossref] [Google Scholar] [PubMed]
- Dehghanizade S, Arasteh J, Mirzaie A. Green synthesis of silver nanoparticles using Anthemis atropatana extract: Characterization and in vitro biological activities. Artif Cells Nanomed Biotechnol 2018;46(1):160-8.
[Crossref] [Google Scholar] [PubMed]
- Moteriya P, Chanda S. Synthesis and characterization of silver nanoparticles using Caesalpinia pulcherrima flower extract and assessment of their in vitro antimicrobial, antioxidant, cytotoxic, and genotoxic activities. Artif Cells Nanomed Biotechnol 2017;45(8):1556-67.
[Crossref] [Google Scholar] [PubMed]
- Fouad H, Hongjie L, Hosni D, Wei J, Abbas G, Ga’al H, et al. Controlling Aedes albopictus and Culex pipiens pallens using silver nanoparticles synthesized from aqueous extract of Cassia fistula fruit pulp and its mode of action. Artif Cells Nanomed Biotechnol 2018;46(3):558-67.
[Crossref] [Google Scholar] [PubMed]
- Singh H, Du J, Singh P, Yi TH. Ecofriendly synthesis of silver and gold nanoparticles by Euphrasia officinalis leaf extract and its biomedical applications. Artif Cell Nanomed Biotechnol 2018;46(6):1163-70.
[Crossref] [Google Scholar] [PubMed]
- Huo Y, Singh P, Kim YJ, Soshnikova V, Kang J, Markus J, et al. Biological synthesis of gold and silver chloride nanoparticles by Glycyrrhiza uralensis and in vitro applications. Artif Cells Nanomed Biotechnol 2018;46(2):303-12.
[Crossref] [Google Scholar] [PubMed]
- Vijayan R, Joseph S, Mathew B. Indigofera tinctoria leaf extract mediated green synthesis of silver and gold nanoparticles and assessment of their anticancer, antimicrobial, antioxidant and catalytic properties. Artif Cells Nanomed Biotechnol 2018;46(4):861-71.
[Crossref] [Google Scholar] [PubMed]
- Zia G, Sadia H, Nazir S, Ejaz K, Ali S, Iqbal T, et al. In vitro studies on cytotoxic, DNA protecting, antibiofilm and antibacterial effects of biogenic silver nanoparticles prepared with Bergenia ciliata rhizome extract. Curr Pharm Biotechnol 2018;19(1):68-78.
[Crossref] [Google Scholar] [PubMed]
- Pavithra Bharathi V, Ragavendran C, Murugan N, Natarajan D. Ipomoea batatas (Convolvulaceae)-mediated synthesis of silver nanoparticles for controlling mosquito vectors of Aedes albopictus, Anopheles stephensi, and Culex quinquefasciatus (Diptera: Culicidae). Artif Cell Nanomed Biotechnol 2017;45(8):1568-80.
[Crossref] [Google Scholar] [PubMed]
- Ozougwu JC, Eyo JE. Studies on the anti-diabetic activity of Allium sativum (garlic) aqueous extracts on alloxan-induced diabetic albino rat. Pharmacologyonline 2010;2:1079-88.
- Waheed A, Nawaz U, Miana GA. Antidiabetic actions of powdered plant and aqueous extract of Allium sativum (garlic) bulbs in type-II diabetic patients. Med Forum Monthly 2014;25(8):27-31.
- Padiya R, K Banerjee S. Garlic as an anti-diabetic agent: Recent progress and patent reviews. Recent Pat Food Nutr Agric 2013;5(2):105-27.
[Crossref] [Google Scholar] [PubMed]
- Thomson M, Al-Qattan KK, Js D, Ali M. Anti-diabetic and anti-oxidant potential of aged garlic extract in streptozotocin-induced diabetic rats. BMC Complement Altern Med 2015;16(1):17.
[Crossref] [Google Scholar] [PubMed]
- Kalhotra P, Chittepu VC, Osorio-Revilla G, Gallardo-Velazquez T. Phytochemicals in garlic extract inhibit therapeutic enzyme DPP-4 and induce skeletal muscle cell proliferation: A possible mechanism of action to benefit the treatment of diabetes mellitus. Biomolecules 2020;10(2):305.
[Crossref] [Google Scholar] [PubMed]
- Kalhotra P, Chittepu VC, Osorio-Revilla G, Gallardo-Velazquez T. Phytochemicals in garlic extract inhibit therapeutic enzyme DPP-4 and induce skeletal muscle cell proliferation: A possible mechanism of action to benefit the treatment of diabetes mellitus. Biomolecules 2020;10(2):305.
[Crossref] [Google Scholar] [PubMed]
- Otunola GA, Afolayan AJ, Ajayi EO, Odeyemi SW. Characterization, antibacterial and antioxidant properties of silver nanoparticles synthesized from aqueous extracts of Allium sativum, Zingiber officinale, and Capsicum frutescens. Pharmacogn Magazine 2017;13(Suppl 2):S201.
[Crossref] [Google Scholar] [PubMed]
- Andleeb S, Tariq F, Muneer A, Nazir T, Shahid B, Latif Z, et al. In vitro bactericidal, antidiabetic, cytotoxic, anticoagulant, and hemolytic effect of green-synthesized silver nanoparticles using Allium sativum clove extract incubated at various temperatures. Green Proc Synt 2020;9(1):538-53.
- Ahmed SR, Roy R, Romi IJ, Hasan M, Bhuiyan MK, Khan MM. Phytochemical screening, antioxidant and antibacterial activity of some medicinal plants grown in Sylhet region. IOSR J Pharm Biol Sci 2019;14(1):26-37.
- Khan MZ, Tareq FK, Hossen MA, Roki MN. Green synthesis and characterization of silver nanoparticles using Coriandrum sativum leaf extract. J Engin Sci Technol 2018;13(1):158-66.
- Eze ED, Mohammed A, Musa KY, Tanko Y, Isa AS. Effect of ethanolic leaf extract of" mucunapruriens"(fabaceae) on lipid profile inalloxan-induced diabetic wistar rats. Br J Pharmacol Toxicol 2012;3(3):102-9.
- Snedecor GW, Cochran WG. Statistical methods, 8th ed, Iowa State University Press, Ames; 1994.
- Krystosiak P, Tomaszewski W, Megiel E. High-density polystyrene-grafted silver nanoparticles and their use in the preparation of nanocomposites with antibacterial properties. J Colloid Interface Sci 2017;498:9-21.
[Crossref] [Google Scholar] [PubMed]
- Elamawi RM, Al-Harbi RE, Hendi AA. Biosynthesis and characterization of silver nanoparticles using Trichoderma longibrachiatum and their effect on phytopathogenic fungi. Egyptian J Biol Pest Control 2018;28(1):1-1.
- Veerasamy R, Xin TZ, Gunasagaran S, Xiang TF, Yang EF, Jeyakumar N, et al. Biosynthesis of silver nanoparticles using mangosteen leaf extract and evaluation of their antimicrobial activities. J Saudi Chem Soc 2011;15(2):113-20.
- El-Refai AA, Ghoniem GA, El-Khateeb AY, Hassaan MM. Eco-friendly synthesis of metal nanoparticles using ginger and garlic extracts as biocompatible novel antioxidant and antimicrobial agents. J Nanostruct Chem 2018;8(1):71-81.
- Von White G, Kerscher P, Brown RM, Morella JD, McAllister W, Dean D, et al. Green synthesis of robust, biocompatible silver nanoparticles using garlic extract. J Nanomater 2012;2012(1):730746.
[Crossref] [Google Scholar] [PubMed]
- Ali IA, Ahmed AB, Al-Ahmed HI. Green synthesis and characterization of silver nanoparticles for reducing the damage to sperm parameters in diabetic compared to metformin. Sci Rep 2023;13(1):2256.
[Crossref] [Google Scholar] [PubMed]
- Nakagawa S, Masamoto K, Sumiyoshi H, Harada H. Acute toxicity test of garlic extract. J Toxicol Sci 1984;9(1):57-60.
[Crossref] [Google Scholar] [PubMed]
- Gaillet S, Rouanet JM. Silver nanoparticles: Their potential toxic effects after oral exposure and underlying mechanisms–a review. Food Chem Toxicol 2015;77:58-63.
[Crossref] [Google Scholar] [PubMed]
- Mahmood S, Talat A, Karim S, Khurshid R, Zia A. Effect of cinnamon extract on blood glucose level and lipid profile in alloxan induced diabetic rats. Pak J Physiol 2011;7(1):13-6.
- Tanko Y, Halidu A, Mohammed A, Ahmed MK, Musa KY. Effect of ethanol extract of Caralluma dalzielii NE Br.(Asclepiadaceae) on blood glucose levels of fructoseinduced insulin resistance in laboratory. Ann Biol Res 2012;3:4980.
- Jini D, Sharmila S, Anitha A, Pandian M, Rajapaksha RM. In vitro and in silico studies of silver nanoparticles (AgNPs) from Allium sativum against diabetes. Sci Rep 2022;12(1):22109.
[Crossref] [Google Scholar] [PubMed]
- Anwar S, Desai S, Eidi M, Eidi A. Antidiabetic activities of Fenugreek (Trigonella foenum-graecum) Seeds. Ed. Victor R. Preedy, Ronald Ross Watson, Vinood B. Patel. Nuts and Seeds in health and disease prevention. Academic Press; 2011. p. 469-78.
- Keshetty V, Pabba S, Gudipati R, Kandukuri JM, Allenki V. Antihyperlipidemic Activity of methanolic extract of Garlic (Allium sativum L.) in Triton X-100 induced hyperlipidemic rats. J Pharm Res 2009;2(5):23-31.
- Manam VK, Subbaiah M. In-vivo anti-diabetic efficacy of silver nanoparticles from marine brown seaweed Colpomenia sinuosa on Alloxan stimulated hyperglycemic activity in wistar albino rats. J Nanosci Technol 2022;8:956-9.
- Nofer JR, Kehrel B, Fobker M, Levkau B, Assmann G, von Eckardstein A. HDL and arteriosclerosis: Beyond reverse cholesterol transport. Atherosclerosis 2002;161(1):1-6.
[Crossref] [Google Scholar] [PubMed]
- Mathew BC, Biju RS. Neuroprotective effects of garlic a review. Libyan J Med 2008;3(1):23-33.
[Crossref] [Google Scholar] [PubMed]
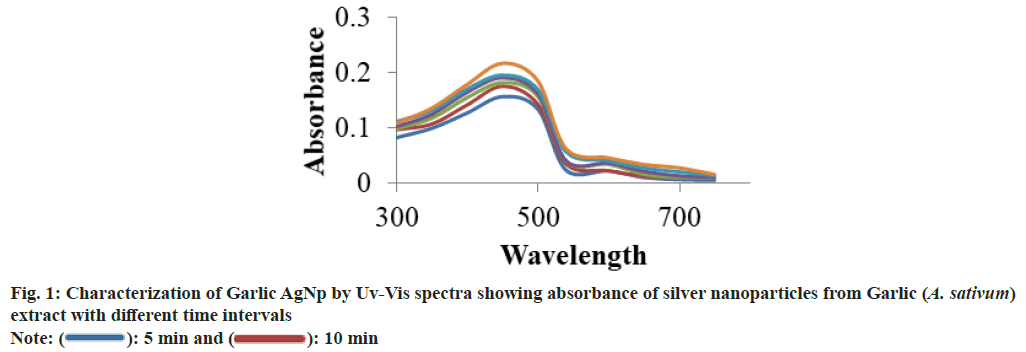
 ): 5 min and (
): 5 min and ( ): 10 min
): 10 min