- *Corresponding Author:
- P. R. Murumkar
G. H. Patel Pharmacy Building, Opp. M. S. University Main Offi ce, Donors Plaza, Fatehganj, Vadodara - 390 002, India
E-mail: prashant_murumkar@yahoo.com
| Date of Submission | 23 March 2006 |
| Date of Revision | 23 March 2007 |
| Date of Acceptance | 18 August 2007 |
| Indian J Pharm Sci, 2007, 69 (4): 605-608 |
Abstract
A series of nicotinyl 4-oxathieno[2,3- d ]pyridine-2-ylmethyl esters, title compounds were synthesized. Compound IIIa was subjected to in vivo antihyperlipidemic activity in Wistar rats. The activity exhibited by synthesized compound was found to be less as compared to that produced by a standard, atorvastatin, administered in the form of suspension in 2% gum acacia. The activity was even lesser than that produced by the lead IIa.
Nicotinic acid can be taken for longer periods without serious toxicity, for plasma lipid lowering [1]. Even studies indicated that nicotinic acid can be added to the lipid lowering action of other drugs, particularly clofibrate and bile acid-binding resigns [2]. Although, some success has been achieved in reducing cardiovascular risk by decreasing blood cholesterol level, still emphasis need to be given on the fact that cholesterol is only a secondary risk factor. The primary risk factor for cardiovascular diseases is the weakness and instability of blood vessel wall which is unfortunately not considered for decreasing cardiovascular risk. Conventional medicine is limited only to treating the symptoms of secondary risk factors. As per modern cellular medicine cholesterol, triglycerides, low density lipoproteins (LDL), lipoprotein (a) and other metabolic products are actually ideal repair factors and their blood levels increase in response to the weakness of the arterial walls. A chronic weakness of the blood vessel walls increases the demand and thereby increases the production rates of these repair molecules in the liver, this leads to increased level of cholesterol and other repair factors in the blood stream and over some time renders them risk factors for cardiovascular disease [3]. Thus the primary measure for lowering cholesterol and other risk factors in the blood stream is to stabilize the arterial wall and thereby decrease the metabolic demand for increased production of these risk factors.
Further cholesterol lowering without first stabilizing the arterial wall may be an insufficient cardiovascular therapy.
Scientific research and clinical studies have already documented the particular value of vitamin B3 (nicotinic acid) and also vitamins like vitamin C, vitamin B5 (pantothenic acid), vitamin E and carnitine in lowering of elevated cholesterol levels and other secondary risk factors in the blood. Vitamins and other essential nutrients lower the particular rate of cholesterol and other repair molecules in the liver and at the same time, contribute to the repair of the arterial wall.
These facts ignited a thought to develop novel molecules which are basically comprising of both, vitamin B3 as well as 2-chloromethylthienopyrimidine- 4(3H)-one nucleus, the later having already demonstrated significant potential to reduce blood cholesterol levels [4-7]. Thus the aim of present work was to synthesize the mutual prodrugs and evaluates it in Wistar albino rats for antihyperlipidemic activity.
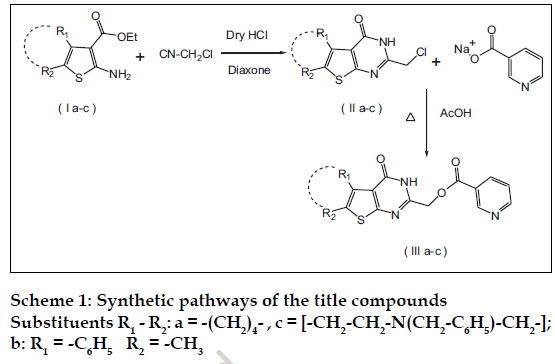
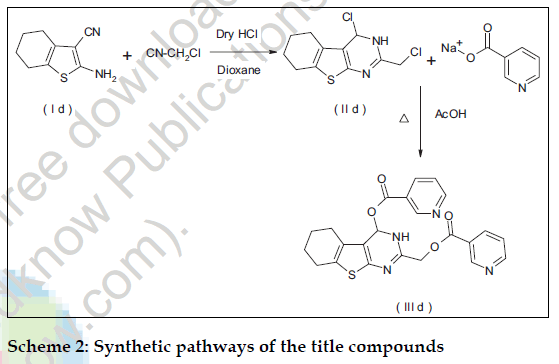
All the chemicals used in the synthesis were of laboratory grade. The Melting points were determined in open capillary method on Campbel electronic apparatus and are uncorrected. The ultraviolet absorption spectra were determined in methanol by using a Shimadzu UV/Vis 1601 double beam spectrophotometer. The IR spectra of synthesized compounds were recorded on Shimadzu 8400-S FT-IR in potassium bromide discs. The 1H NMR spectra were recorded in CDCl3 using NMR Varian-Mercury 300 MHz spectrometer and chemical shifts are given in units as δ ppm, downfield from tetramethylsilane (TMS) as an internal standard. Mass spectra were obtained on an Electron Impact mass spectrometer at 70 eV ionizing beam and using direct insertion probe. The spectral data of the synthesized compounds is shown in Table 1. Thin layer chromatography was performed on microscopic slides (2×7.5 cm) coated with silica gel-G, using benzene-methanol or benzene-methanol-ammonia as the solvent systems and the spots were visualized by exposure to iodine vapors or under UV light. The titled compounds were synthesized as per scheme-1 and scheme-2.
| Compound | UV(MeOH) λmax (nm) | IR (KBr) cm-1 | PMR (CDCl3) | Mass |
|---|---|---|---|---|
| IIa | 317 | 1664, 3015, 937, 792, | 1.62-1.99 (4H, m), | 255(M+) |
| 754, 686. | 2.78-3.02 (4H, m), 4.56 | |||
| IIb | 316 | (2H, s). | ||
| 1658 | 2.40 (3H, s), 4.42 (2H, s), | - | ||
| IIc | 326.4 | 7.38-7.44( 5H, m). | ||
| 1666,3028, 2847, | 1.25-1.41(2H,m ), | - | ||
| 1.42-1.59 (2H, s), | ||||
| 744, 696 | 2.94- 3.18 (2H, m) 3.74-3.81, | |||
| IId | 301 | 4.54 (2H, m), 7.40-7.61 (2H, s) | ||
| 1.25-1.41(2H, m), 1.42-1.59 (2H, s), | - | |||
| 936, 736 | 2.94- 3.18( 2H, m), 3.74-3.81, | |||
| IIIa | - | 4.54 (2H, m), 7.40-7.61 (2H, s) | ||
| 1680, 1630 ,1580, 2937 | 1.7-1.8 (4H, m), 2.6-2.8, | - | ||
| (4H, m), 5.85 s, , 8.1, m, 4, 9.5, m, | ||||
| IIIb | - | 1687, 1639,1581, 2947 | - | - |
| IIIc | - | 1674, 1635,1587, 2937 | - | - |
| IIId | - | 1678, 1647,1584, 2931 | - | - |
Table 1: Spectral Data
Nicotinic acid-4-oxo-3,4,5,6,7,8-hexahydrobenzo [4,5 ]thieno [2,3-d] pyrimidin-2-ylmethyl ester (IIIa) was synthesized by bubbling a stream of dry hydrogen chloride gas through an ice-cold mixture of 2-amino- 3-carbethoxy-4,5,6,7-tetrahydrobenzo(b)thiophene (Ia), (13.52 g; 0.06 mole) and chloroacetonitrile (6.8 g; 0.09 mole) in dry dioxane (60 ml) for 6 h at temperature below 10°. The reaction mixture was allowed to stand thereafter at room temperature for 12 h. Then it was heated on a water bath for 2 to 3 h, cooled to room temperature and poured into ice-water mixture (150-200 ml) and neutralized with dilute ammonia. The solid separated was filtered, washed with water and dried. The crude product on crystallization from dioxane yielded fine needles (14.15 g; 92.7%), mp: 275-277º (273-276º)4 characterized as 2-chloromethyl- 5,6,7,8-tetrahydrobenzo(b)thieno- [2,3-d]pyrimidin- 4(3H)-one (IIa). A solution of (11a), (1.5g; 0.006 mol) and sodium nicotinate (2.93g; 0.024 mol) in glacial acetic acid (25 ml) was refluxed for 12 h, poured into ice-water and basified with dilute ammonia. The precipitate formed was filtered, dried and crystallized from acetic acid to obtain light yellow powder (1.24 g; 61.2% yield), mp: 296-298º , characterized as (IIIa).
Nicotinic acid-4-oxo-6-methyl-5-phenylthienothieno [2,3- d] pyrimidin-2-ylmethyl ester (IIIb) was synthesized using the following procedure; 2-amino-3-carbethoxy- 5-methyl-4-phenylthiophene (15.68 g; 0.06 mole) and chloroacetonitrile (6.7 g; 0.09 mole) were treated in dry dioxane (60 ml) as per the procedure described for (IIa) and crystallized from ethanol-chloroform (15.04 g; 86.3%), mp: 261-264 º, characterized as 2- chloromethyl-6-methyl-5-phenylthieno [2,3-d]pyrimidin- 4(3H)-one (IIb). A solution of (11b, 1.5 g; 0.005 mol) and sodium nicotinate (2.88 g; 0.02 mol) in glacial acetic acid (25 ml) was refiuxed for 28 h as per the above procedure and crystallized from methanolchloroform yielded yellow powder (1.9 g; 92.72% yield), mp: 230-232º, characterized as (IIIb).
Nicotinic acid-4-oxo-7-Benzyl-2-Chloromethyl- 5,6,7,8-tetrahydro-(3H)-pyrido-4’,3’:4,5]thieno [2,3 -d]- pyrimidin-2-ylmethyl ester( IIIc) was prepared as follows; 1-benzyl-4-piperidone (18.92 g; 0.1 mole), ethyl cyanoacetate (11.3 g; 0.1 mole), and sulfur (3.2 g; 0.1 mole) were dissolved in ethanol (20 ml). To this well stirred mixture, diethylamine (9.14 g; 0.125 mole) was added drop wise for ½ hour and stirring continued for another 3 h at ambient temperature. The reaction mixture was kept in refrigerator overnight. The solid separated was filtered and washed with 20 ml chilled 50% aqueous methanol to yield (21.6g; 68.2%), mp; 98-100° characterized as 2-amino-6-benzyl-3-carbethoxy- 4,5,6,7-tetrahydrothieno [2,3-c]pyridine (Ic).
The compound (Ic) (18.98 g; 0.06 mole) was treated with chloroacetonitrile (6.7 g; 0.09 mole) in dry dioxane (60 ml) as per the procedure described for compound (IIa). The crude product on crystallization from ethanol-chloroform yielded (16.17g; 78.0%), mp: 232-234º, characterized as 7-Benzyl-2-chloromethyl- 5,6,7,8–tetrahydro-3H-pyrido- [4’,3’:4,5]thieno [2,3- d]pyrimidine-4-one (IIC). A solution of (IIc, 2.0g; 0.006 mol) and sodium nicotinate (3.45g; 0.02 mol) were treated in glacial acetic acid (25 ml) for 28 hours as per the procedure described for (IIIa) and crystallized from methanol-chloroform yielded light yellow powder (1.5 g; 80.25% yield), mp: 270-272°, characterized as (IIIc).
Dinicotinic acid--6-methyl-5-phenylthieno [2,3-d] pyrimidin-2,4-ylmethyl ester (IIId) was synthesized by adding to the solution of cyclohexanone (9.8g; 0.1mole) in ethanol sulfur (3.2 g; 0.1 mole), malonitrile (6.6g, 0.1mole) and diethylamine (9.14 g; 0.125 mole) and the reaction was carried out as per the procedure described for (Ia) to yield 2-amino- 3-cyano-4,5,6,7-tetrahydrobenzo(b) thiophene (Id) The compound (Id) (3.6g; 0.02 mole) and chloroacetonitrile (2.0g; 0.03 mole) were treated in dry dioxane (60 ml) as per the procedure described for (IIa) and crystallized from dioxane afforded (5.5g; 72.72%), mp: 85-87º, characterized as 2- chloromethyl-4-chloro-5,6,7,8 tetrahydrobenzo(b)thie no [2-3-d]pyrimidine (II d). A solution of Compound (IId) (6.0g; 0.02 mol) and sodium nicotinate (5.8g; 0.04 mol) were treated in dimethylformamide (25 ml) for 14 h as per the procedure described for (IIIa) and crystallized from methanol-chloroform (7.0g; 73.0%), mp: 150-152º, characterized as (IIId).
The 2-chloromethyl-5,6,7,8-tetrahydro-benzo(b)thieno [2,3- d]-pyrimidine-4(3H)-one (IIa) and the mutual prodrug, nicotinic acid-4-oxo-3,4,5,6,7,8-hexahydrobenzo [4, 5]thieno [2,3-d]pyrimidine-2-yl methyl ester (IIIa) were screened for a two week preventive study in cholesterol diet fed hyperlipidaemic Wistar rats using atorvastatin as standard. The results were evaluated in term of % reduction in plasma cholesterol levels.
The activities exhibited by the synthesized compounds (Table 2) were found to be less as compared to that produced by a standard, atorvastatin, administered in the form of suspension in 2% gum acacia. The total serum cholesterol reduced by the standard drug, atorvastatin, in the experimental animals were 49±2.53%. Against this, the compounds (IIa) and (IIIa) reduced the total serum cholesterol by 39.6±4.70 and 14.08±4.19%, respectively.
| Compound | Cholesterol level inserum (mg/dl) | % Decrease inserum cholesterol level | |
|---|---|---|---|
| Initial | Final | ||
| Cholesterol control | 62.95 | 74.5 | 39.60±5.70 |
| IIa | 75.84 | 54.35 | |
| IIIa | 61.24 | 62.09 | 14.08±4.19 |
| Atorvastatin | 66.12 | 47.09 | 49.81±2.53* |
*Results are expressed as mean ± standard error, statistically significant (P<0.05, t test, n=6)
Table 2: Antihyperlipidemic Activity
Acknowledgements
Authors are thankful to Dr. Jayant S. Kulkarni, Former Head, of Department of Pharmacology, R.C. Patel College of Pharmacy, Shirpur for providing Wistar rats and necessary facility to carry out pharmacological activity.
References
- The Coronary Drug Project Research group, Clofibraten and niacin in coronary heart disease, J. Amer. Med. Assoc., 1975, 231, 360.
- Grundy, S.M., Henry, Y.I., Mock and Zech, L., J. Lipid Research.,1981, 22, 307.
- Rath, M., .Why Animals Don.t Get Heart Attack But People Do!., 4th Edn, MR publishing, Inc., Fremont, CA,USA,2003, 80.
- Raab, J. Indian Chem. Soc., 1946, 23, 276.
- Askelof, E.E., U S Patent 2396145, 1946.
- Chourasia, K.K. and Jain, S.K, J. Pharm. Sci., 2003, 6, 33.
- Shishoo, C.J., Jain, K.S., Rathod, I.S., Thakkar, B.J., Brahmabhatt, S.B., Gandhi, T.P., Bangaru, R., Arzneim. Forsch /Drug Res., 1996, 46, 273.
- Gewald, K, Angew Chem.,1961,73,114.
- Gewald, K., Chem. Ber., 1965, 98, 3571.
- Gawald K., Schinke,E., Bottcher, H., Chem. Ber., 1966, 99, 94.
- Gewald, K., Z. Chem. Ber., 1963, 22, 305.
- Shishoo,C.J,. Devani, M.B., Pathak, U.S., Ananthan, S., Bhadti, V.S.and Ullas, G.V., J. Het. Chem, 1984, 21, 375.
- Shishoo C.J., Devani M.B., Bhadti V.S., Jain K.S., Goyal R.K. and Naik S.R. Arzneim. Forsch /Drug Res., 1990, 40, 56.
- Shishoo C.J., Jain, K.S., Rathod I.S., Thakkar B.J., Brahmabhatt S.B. and Gandhi T.P., Arzneim. Forsch/Drug Res., 1996, 46, 273.