- *Corresponding Author:
- Boyang Liu
Department of Clinical Medicine,
College of Medicine,
Zhejiang University,
Hangzhou, Zhejiang Province 310020,
P. R. China
E-mail: 3180105305@zju.edu.cn
| This article was originally published in a special issue, “New Advancements in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(2) Spl Issue “217-225” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Biatractylolide is an Atractylodes macrocephala derived bisesquiterpenoid that has been found treatment of dementia in model mice. This study aims to explore the therapeutic effect on vascular dementia. Network pharmacological techniques have been used to elaborate the involved mechanisms of biatractylolide treating vascular dementia. Based on PubChem database, Swiss target prediction and human gene database GeneCards, 19 common target molecules were screened, they were enriched in many biological, such as Alzheimer’s disease corrective pathway, pathways of neurodegeneration-multiple diseases, calcium signaling pathway, notch signaling pathway and vascular endothelial growth factor signaling pathway. Muscarinic acetylcholine receptor M1, amyloid-beta precursor protein, microtubule-associated protein tau, presenilin-1, presenilin-2, prostaglandin G/H synthase 2 and disintegrin, and metalloproteinase domain-containing protein 17 were enriched in Alzheimer’s disease corrective pathway. Protein–protein interaction network presents that six highest degree nodes, phosphoinositide-3-kinase regulatory subunit 1, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha, Harvey rat sarcoma virus, Jun N-terminal kinases, catenin beta 1, mitogen-activated protein kinase 3 and tumor protein P53, play core role in vascular dementia and four molecules, mitogen-activated protein kinase 14, Janus kinase 2, amyloid-beta precursor protein and tyrosine-protein phosphatase non-receptor type 1, closely interact with biatractylolide. In addition, acetylcholinesterase was identified to have a strong interaction with biatractylolide. This study suggests that biatractylolide is a potential drug for treatment of vascular dementia or Alzheimer’s disease.
Keywords
Biatractylolide, vascular dementia, systematic pharmacology, antioxidation
Biatractylolide (BA) is a bisesquiterpenoid isolated from traditional Chinese herb Atractylodes macrocephala that is a folk medicine for treatment of gastroenteric and splenic disorders [1]. Various activities of the herbal plant, including antioxidation, gastroprotection, antitumor and anti-Alzheimer’s disease [2], should be attributed to its active ingredients, such as BA that has been unraveled an obvious inhibition effect on Acetylcholinesterase (AChE) in brain of aluminum trichloride induced dementia mice and improving memory ability of the mice [3]. Also, it has been observed in Amyloid beta peptide (Aβ1–40) induced Alzheimer’s Disease (AD) model rats that BA inhibited Cholinesterase (ChE) activity and improved behavior and memory [4]. Note worthily, both activity and expression of AChE were dose-dependently repressed by BA [5]. These pharmacological effects were correlated with the mechanism of Phosphatidylinositol 3 Kinase-Protein Kinase B-Glycogen Synthase Kinase 3 beta (PI3K-Akt-GSK3β) dependent pathways. In Aβ-induced AD rats, it has been also observed that BA reduced apoptosis of hippocampal cells, prevented cognitive impairment and morphological changes of hippocampal nerve cells via inhibiting activation of Nuclear Factor kappa B (NF-κB) signal pathway [6]. These findings suggest that BA should be beneficial for dementia therapy.
Dementia is a type of serve neurodegenerative diseases that are characterized by memory loss, thinking impairments and decline of daily living activities, number of cases approximately doubles every 5 y in the geographic region including Asia, Africa, South America, North America and Europe from January 1985 to August 2019. Vascular Dementia (VaD) is the second common dementia next to AD, but it is not easy to differentiate between AD and VaD because of their overlapped features of symptomatology and pathophysiology, and risk factors [7]. In VaD cases aged 60 y to 69 y, number of males is 1.8 times of females, prevalence rate is higher in Europe and North America than in Asia, Africa and South America. In mainland China from 2009 to 2019, it has been observed a higher VaD prevalence in northeast China population, in urban and males, and an increasing prevalence with age [8].
Currently no standard treatments have been approved for VaD, the clinical drugs for VaD are only suitable for symptomatic therapy and prevent or delay the course of VaD [9]. Two important features, inflammatory responses and cholinergic dysfunction, has been considered to be treatment targets for VaD [10]. Recently, our unpublished preliminary research unraveled that BA administration effectively repressed development of pathological features in brain of VaD C57BL/6 mice based on histopathological study. To further understand the pharmacological effect of BA on VaD, this investigation predicted the possible involved mechanisms based on network pharmacological strategy.
Materials and Methods
Targets identification of ba anti-vad:
The sdf file with BA Three-Dimensional (3D) structure was downloaded from the PubChem database (https:// pubchem.ncbi.nlm.nih.gov/). Swiss Target Prediction platform (http://www.swisstargetprediction.ch/index. php) was used to search BA targets in Homo sapiens based on 3D similarity of chemical structure. Moreover, The PharmMapper platform (http://www.lilab-ecust. cn/pharmmapper/) was used to capture potential BA targets through pharmacophore mapping approach. The duplication was eliminated and the targets collected by the two prediction methods were merged.
Based on Medical Subject Headings (MeSH) of VaD, namely dementia, vascular and related disease genes retrieved on DisGeNET and GeneCards databases, the same molecular targets between VaD associated target genes and BA target molecules were picked up.
Bioinformatic annotation:
Target proteins of BA were classified in Protein Analysis through Evolutionary Relationships (PANTHER) classification system (http://pantherdb. org/). Furthermore, enrichment analysis of targets of BA anti-VaD was performed in Profiler platform (http:// biit.cs.ut.ee/gprofiler/gost). Key regulatory pathways were mapped in Kyoto Encyclopedia of Genes and Genomes (KEGG) database (https://www.kegg.jp/ kegg/). The interaction between BA affinity targets and VaD-related proteins was analyzed on Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) platform (https://string-db.org/) and a visual network was constructed by Cytoscape software.
Molecular docking:
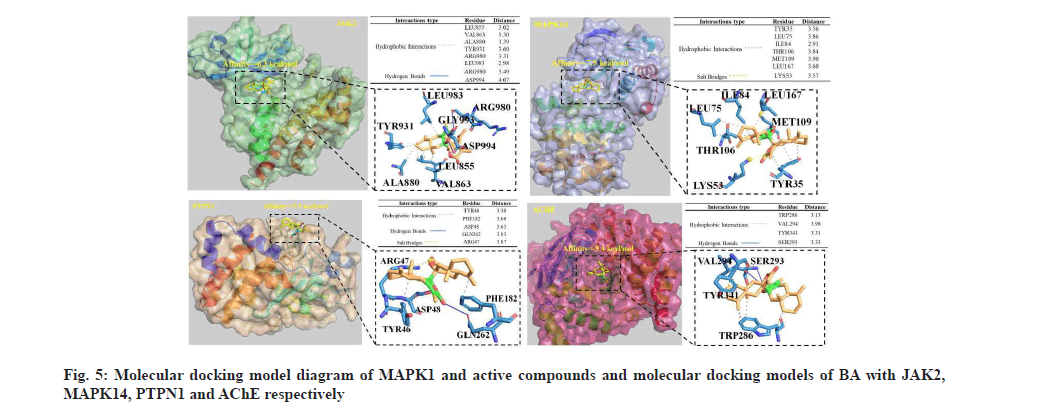
Download crystal structures of the interest BA molecular targets, including Janus Kinase 2 (JAK2) (Protein Database (PDB) ID: 4IVA), Mitogen- Activated Protein Kinase 14 (MAPK14) (PDB ID: 1YQJ), Tyrosine-Protein Phosphatase Non-Receptor Type 1 (PTPN1) (PDB ID: 1C88) and AChE (PDB ID: 6O5V) from the PDB (http://www.rcsb.org/). PyMOL software (The PyMOL Molecular Graphics System, Version 2.0 Schrödinger and LLC) was used to peel the original ligand from the target protein. Ligand and macromolecule files for molecular docking were prepared by AutoDock Tool. Molecular docking is then performed via AutoDock Vina. The docking results were uploaded to Protein Ligand Interaction Profiler (PLIP) platform (https://plip-tool.biotec.tu-dresden.de/ plip-web/plip/index) to analyze the interaction between small molecules and proteins.
Results and Discussion
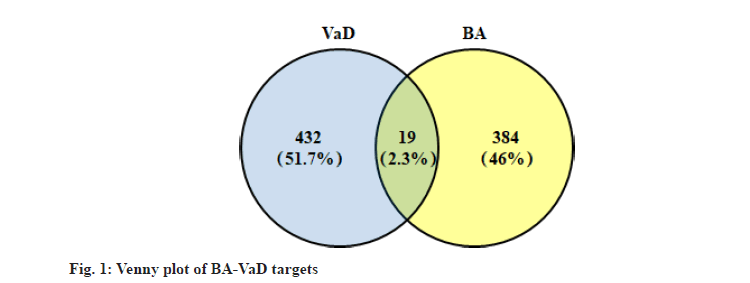
A total of 403 BA affinity targets were captured from PharmMapper and Swiss Target Prediction platform based on cheminformatics strategies. 381 VaD-related genes with relevance score greater than 15 were selected from GeneCards databases. After eliminating the duplicates, 451 disease genes were obtained by merging with 208 related entries in DisGeNET database. As shown in the Venn diagram (fig. 1), the intersection of BA targets and VaD-related genes consists of 19 objects. Nearly half of these targets were classified as enzymes as shown in Table 1, including protease (Presenilin-1 (PSEN1), Presenilin-2 (PSEN2), A Disintegrin and Metalloproteinase Domain- Containing Protein 17 (ADAM17)), Phospholipase A2 Group IIA (PLA2G2A), Superoxide Dismutase 2 (SOD2), Myeloperoxidase (MPO), Prostaglandin Endoperoxide Synthase 2 (PTGS2), MAPK14 and PTPN1. Moreover, 5 targets belong to the receptor protein subgroup, including G protein-coupled receptor (Cholinergic Receptor Muscarinic 1 (CHRM1), 5-Hydroxytryptamine Receptor 6 (HTR6), Oxytocin Receptor (OXTR)) and transmembrane signaling receptor (Kinase Insert Domain Receptor (KDR) and Fms Related Receptor Tyrosine Kinase 1 (FLT1)).
| Gene symbol | Target class | Relevance score of VaD |
|---|---|---|
| MAPT | Unclassified protein | 89.83 |
| APP | Protease inhibitor | 77.83 |
| PSEN1 | Aspartic protease | 72.49 |
| PSEN2 | Aspartic protease | 55.31 |
| JAK2 | Unclassified protein | 54 |
| KDR | Transmembrane signal receptor | 53 |
| CETP | Unclassified protein | 46 |
| PLA2G2A | Phospholipase | 45 |
| SOD2 | Oxidoreductase | 32.82 |
| MPO | Peroxidase | 32 |
| PTGS2 | Oxygenase | 22.79 |
| MAPK14 | Non-receptor serine/threonine protein kinase | 22.61 |
| TSPO | Unclassified protein | 18.21 |
| CHRM1 | G-protein coupled receptor | 16.31 |
| ADAM17 | Protease | 12.38 |
| HTR6 | G-protein coupled receptor | 8.56 |
| PTPN1 | Protein phosphatase | 3.74 |
| OXTR | G-protein coupled receptor | 3.48 |
| FLT1 | Transmembrane signal receptor | - |
Table 1: BA Associated Target Molecules
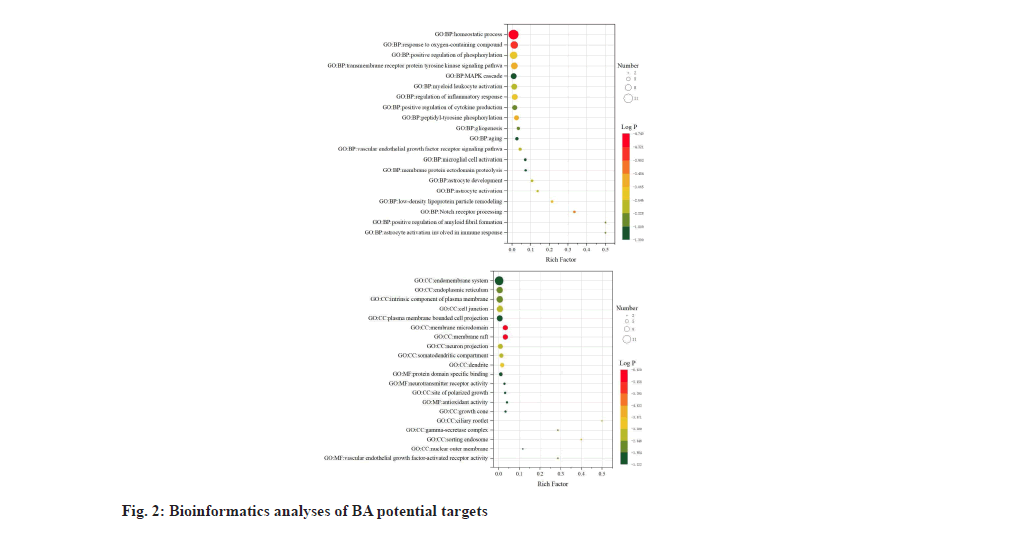
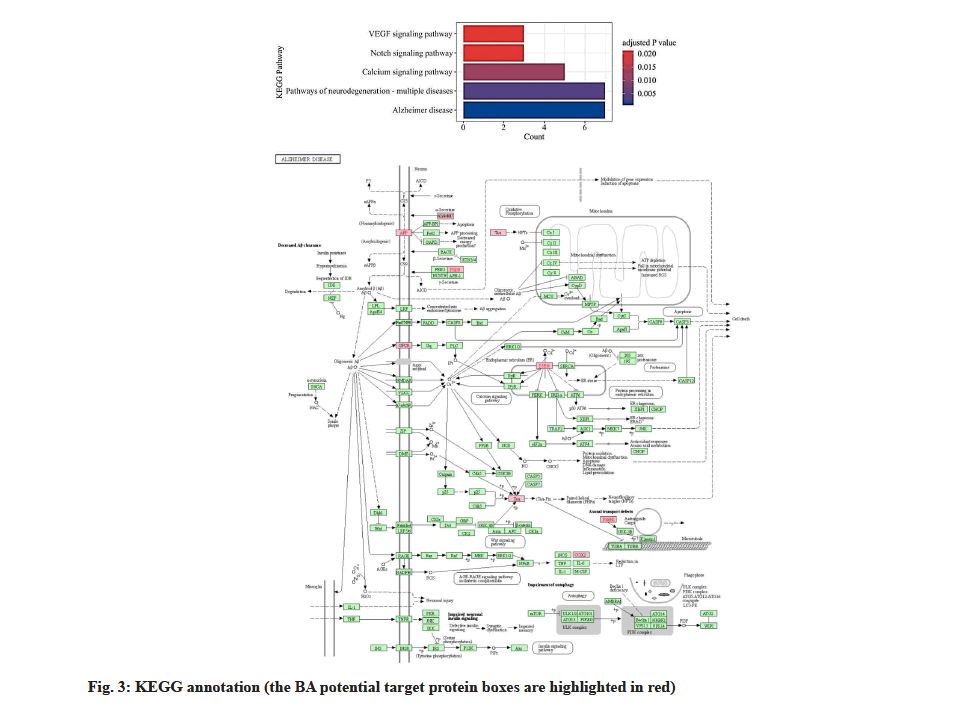
In the enrichment analysis of Biological Processes (BP), BA potential targets had the highest significant level with homeostatic process, with log P value of -4.7 and a total of 12 BA targets were involved as shown in fig. 2. The term astrocyte activation involved in immune response and positive regulation of amyloid fibril formation showed the highest enrichment with a rich factor of 0.5 and the potential BA targets associated with these two processes were Amyloid Precursor Protein (APP) and PSEN1. Membrane micro domain and membrane raft were Cellular Component (CC) terms with the highest level of significance (log P=-6.6) in enrichment analysis. Molecular Functions (MF) of BA anti-VaD targets are mapped to neurotransmitter receptor activity, protein domain specific binding, antioxidant activity and Vascular Endothelial Growth Factor (VEGF)- activated receptor activity. KEGG database showed that 5 signaling pathways matched with intersection targets, among which pathway Alzheimer’s disease (hsa05010) had the highest significant level and 7 binding targets CHRM1, APP, Microtubule-Associated Protein Tau (MAPT), PSEN1, PSEN2, PTGS2 and ADAM17 were included as shown in fig. 3.
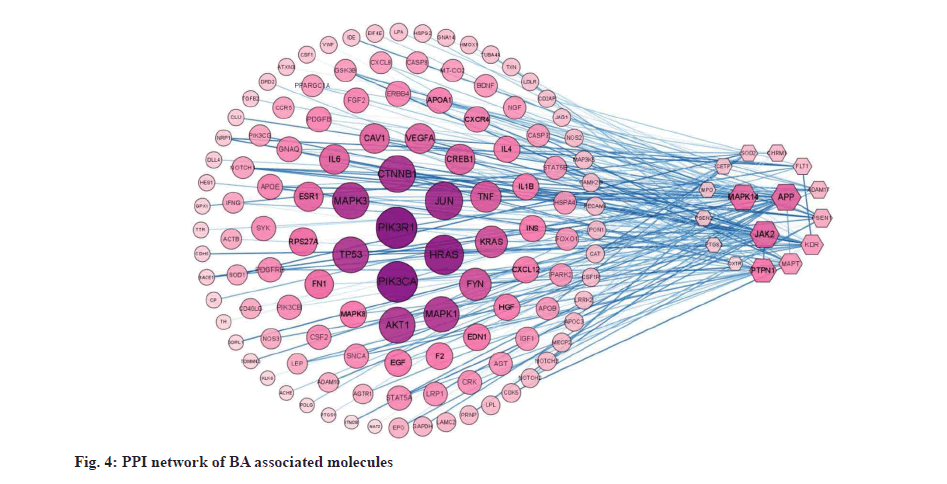
PPI network in fig. 4 shows that the six nodes with the highest degree, including Phosphoinositide-3-Kinase Regulatory Subunit 1 (PIK3R1), Phosphatidylinositol- 4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha (PIK3CA), Harvey Rat Sarcoma virus (HRAS), Jun N-Terminal Kinases (JNKs), Catenin Beta 1 (CTNNB1), Mitogen-Activated Protein Kinase 3 (MAPK3) and Tumor Protein P53 (TP53), play core role in VaD. Among the affinity targets of BA, MAPK14, JAK2, APP and PTPN1 have the largest number of interacting proteins.
Binding capacity of BA to key proteins (JAK2, MAPK14 and PTPN1) in PPI network was evaluated by molecular docking as shown in fig. 5. The binding energies of the small molecules to the three proteins were -6.3, -7.9 and -5.9 kcal/mol, respectively, which were all less than -5.0 kcal/mol, indicating that BA could form stable complexes with these proteins. The interaction between BA and these macromolecules mainly includes hydrophobic interaction, hydrogen bond and salt bridge. AChE that has been reported to be regulated by BA was also tested for molecular docking; the binding energy was -9.4 kcal/mol. In the binding pocket, Ser293 of AChE protein forms hydrogen bonds with BA. Moreover, there was hydrophobic interaction between BA and Trp286, Val294 and Trp341.
As the second most frequent dementia, the main risk factor for VaD is advancing age. Due to lack of effective treatments, rapidly growing VaD cases may result in huge cost in 2050, approaching 4 trillion dollars[11]. Presently, no standard treatment has been approved for VaD. Published pharmacological approaches were on basis on pathophysiological changes of VaD, including inhibition of oxidative stress, restoration of the central cholinergic system, inhibition of neuroinflammation, inhibition of neuronal apoptosis and restoration of synaptic plasticity.
In this work, based on the chemical structure characteristics of BA and VaD associated molecules, 19 potential target molecules were screened out by predictive tools, mainly including 9 enzymes, 5 subgroups of receptor protein and 5 other functional molecules. According to the results of enrichment analysis and PPI network, the molecules function in neurotransmitter receptor activity, protein domain specific binding, antioxidant activity and VEGFactivated receptor activity to play beneficial effect for VaD therapy. Among the five matched signaling pathways, AD associated pathway is the highest association degree, the degeneration disease is the most frequent dementia. Six most relevant molecules of VaD are closely related with four molecules of BA affinity targets, MAPK14, JAK2, APP and PTPN1.
It has been known that neuroinflammation is an important pathological process of VaD, so inhibition of neuroinflammation has been considered as an effective treatment strategy. Neuroinflammatory responses are fundamental to the pathogenesis of many diseases, such as AD and neurodegenerative dementias. JNKs are members of MAPK family, which can be triggered by various stress stimuli. JNK1 and JNK2 express in all cells and tissues throughout the body and JNK3 expresses in heart, brain and testicles [12]. JNKs relate to inflammatory processes in many diseases, including neuroinflammation, mediate NF- κB signaling activation, a major upstream modulator for proinflammatory cytokines [13]. It was found that Interleukin 1 beta (IL-1β) promoted APP expression via activating NF-κB signaling pathway that targeted APP promoter [14-16], moreover IL-1β promoted cleavage of APP via stimulating Gamma secretase (γ-secretase) [17]. Presenilins including PSEN1 and PSEN2, as core components of γ-secretase complex involve in APP cleavage and Aβ release, leading to AD. PSEN1 is the catalytic submit of γ-secretase that is responsible for cleavage of substrates, including APP [18]. Qin et al. found that PSEN2 had a protective effect against Aβ-induced neuroinflammation and brain via suppressing P2X7 expression [19]. Sheng et al. found that neuroinflammation increased APP expression/ processing and caused intracellular accumulation of Aβ [20]. Presently, it is unknown the respective mechanism of the two PSENs on regulating APP processing and the effects of BA on PSENs. In VaD rats, inhibition of mammalian Target of Rapamycin 1 (mTOR1)/NF-κB and JAK2/Signal Transducer and Activator of Transcription 3 (STAT3) pathways and promotion of PI3K/Akt anti-inflammatory pathway could mitigate cognitive deficits. Zhu et al. found that BA could activate PI3K/Akt pathway in glutamate exposed PC12 and SH-SY5Y cells. JNK pathway activation is conducive to inflammatory diseases and neurodegenerative diseases [21,22]. PTPN1, has been identified to be a negative regulator of JNK MAPK pathway via dephosphorylating activated JNK [23]. Consequently, PTPN1 can repress inflammation and neurodegeneration. In our study, a BA can form salt bridges with Arg47, hydrogen bonds with Asp48and Gln262, and hydrophobically interacts with Tyr46 and Phe182 of PTPN1 protein. Also, Arg980 and Asp994 of JAK2 protein form hydrogen bonds with BA and there was hydrophobic interaction between BA and Leu855, Val863, Ala880, Tyr931, Arg880 and Leu983. It can be deduced that BA should be influence on VaD, which is needed to be testified by further study. These results indicated that BA can inhibit neuroinflammation through activating PI3K/Akt/mTOR pathway and then repressing NF-κB and JAK2/STAT3 pathways to suppress production of pro-inflammatory factors and APP.
It is well-known that oxidative stress can induce oxidative damage, also mediate inflammatory response. Reactive Oxygen Species (ROS), an intermediate product of lipid, disorders structure and function of membrane, resulting in cytotoxicity. In VaD animal models, ROS is an important inducer of Nod-Like Receptor Protein 3 (NLRP3) inflammasome activation that leads to neuroinflammation and neurodegeneration [24]. In the work by Zhu et al. BA was also found declined ability of ROS, indicating that BA not only protects brain from ROS mediated oxidative damage, also prevents ROS induced neuroinflammation. Inhibition of oxidative stress has also been considered as an effective strategy for VaD.
Brain damage and cognitive dysfunction induced by neuroinflammation and brain metabolic disorders are partly attributed to excessive production of MAPT encoded tau-protein [25-28]. Tau-protein is mainly expressed in neuronal cells and has many biological functions. There is a close relationship between tau-protein dysmetabolism and neuroinflammation. Genetic or environmental factors activated tau kinase hyper-phosphorylates tau via Cyclin-Dependent Kinase (CDK) 5/p38 MAPK/Calcium-Dependent Protein Kinase Type II Subunit alpha (CAMKIIα) pathway, resulting in tau aggregation to form tau inclusions that in turn activate glia to be reactive microglia/astrocytes, releasing proinflammatory factors, Tumor Necrosis Factor alpha (TNFα) and IL-1β [29]. Although it is unclear for us whether BA performs anti-neuroinflammation via regulating tau-protein, it can be predicted that BA may be involved in tau pathologies to prevent neuroinflammation response.
PTGS2, also called Cyclooxygenase 2 (COX-2), is a critical enzyme that generates prostaglandins to regulate vascular tone, thrombosis, inflammation and pain. PTGS2 is an inducible enzyme that can be induced to high levels during inflammation, which is initiated by several transcription factors associated with inflammatory signal pathways, including NF-κB, AP- 1, Sp1 and CCAAT Enhancer Binding Protein (C/EBP) [30]. Since BA can inhibit activation of NF-κB pathway, expression induction of PTGS2 should be repressed, resulting in production reduction of prostaglandins and consequent alleviation of neuroinflammation.
Cholesteryl Ester Transfer Protein (CETP) is a member of Lipid Transfer/Lipopolysaccharides (LPS) Binding Protein (LT/LBP) gene family, it has been newly known that the protein lacks LPS transfer activity but worsens inflammation [31]. Translocator Protein (TSPO) is a mitochondrial transmembrane protein mainly expressed in glia cells in brain and has various biological functions, such as mitochondrial permeability transition pore opening, mitochondrial respiration, apoptosis and inflammation. TSPO has been considered to be a marker for neuroinflammation or microglia activation, so many inhibitors of TSPO have been identified anti-inflammatory effects [32]. It has been in vivo found that BA exposure reduced ROS content, which might be attributed to inhibition of ROS production. TSPO as a partner of voltage dependent anion channel involves in modulating opening of mitochondrial Permeability Transition Pore (mPTP). Based on the reported findings, it can be deduced that the two molecules should be beneficial for VaD therapy via eliminating neuroinflammation.
MAPK14, a serine/threonine kinase involved in inflammatory process in microglia, can be activated by LPS/TLR4 in microglia and in animal brain, and then activating NLRP3 inflammasome [33]. Most of our data show that BA is effective in inhibiting neuroinflammation, restoring function of central nervous system and alleviating VaD, but it is still unable to draw a direct relationship between BA and MAPK14. Moreover, molecular docking analysis presented that BA formed salt bridges with Lys53 of JNK2 protein and hydrophobically interacted with Tyr35, Leu75, Ile84, Thr106, Met109 and Leu167 in the binding pocket. So we can predict that BA may be repressing MAPK14 associated signaling pathway to alleviating neuroinflammation.
Apart from anti-inflammation treatment, restoring cholinergic function and choline metabolism is another treatment strategy for VaD. AChE inhibitors have been used widely to treat AD and also been used as treatment agent for VaD cases based on the similar pathological mechanism cholinergic dysfunction, such as donepezil, galantamine, rivastigmine, Biochanin A (BCA), ruthenium and montelukast. Currently, three reports have shown that BA was an inhibitor of AChE. We found in this work that BA underwent hydrophobic interaction with Trp286, Val294 and Tyr341 and formed hydrogen bonds with Ser293 in AChE protein. Additionally, AChE expression could be downregulated by BA via inhibiting GSK3β activity. These findings reveal that BA can suppress both activity and expression of AChE, consequently, inhibit enzymatic degradation of excitatory neurotransmitter ACh, resulting in elevation of ACh relative content. It is well-recognized that CHRM1 is a G protein coupled receptor widely expressed in central nervous system, ACh is an agonist that activates the receptor to trigger PI3K/Akt signal pathway [34]. Xie et al. unraveled that BA elevated ACh content through inhibiting AChE activity and activated PI3K/Akt pathway, which may be attributed to activation of CHRM1 by elevated ACh. Additionally, PI3K/Akt is a cellular survival signal pathway that down-regulates B-cell Lymphoma-2/Bcl- 2-associated X protein (Bcl-2/BAX) ratio, alleviating neuronal apoptosis. These data suggest that BA should be beneficial for VaD therapy through reducing total activity of AChE and elevating ACh relative content and then activating PI3K/Akt signal pathway, restoring function of the central cholinergic system.
Conflict of interests:
The authors declared no conflicts of interest.
References
- Lin Y, Jin T, Wu X, Huang Z, Fan J, Chan WL. A novel bisesquiterpenoid, biatractylolide, from the Chinese herbal plant Atractylodes macrocephala. J Nat Prod 1997;60(1):27-8.
- Zhu L, Ning N, Li Y, Zhang QF, Xie YC, Irshad M, et al. Biatractylolide modulates Pi3k-Akt-Gsk3β-dependent pathways to protect against glutamate-induced cell damage in pc12 and sh-sy5y cells. Evid Based Complement Alternat Med 2017;2017.
[Crossref] [Google Scholar] [PubMed]
- Liu Y, Liao CM. Effects of bitatractylolide on the AD rats induced by AlCl3. J Hunan Norm Univ Med Sci 2006;3:25-6.
- Feng X, Wang ZL, Lin YC. Biatractylolide’s effect on Aβ1–40-induced dementia model rats. Chin Pharmacol Bull 2009;25(7):949-51.
- Xie YC, Ning N, Zhu L, Li DN, Feng X, Yang XP. Primary investigation for the mechanism of biatractylolide from Atractylodis Macrocephalae rhizoma as an acetylcholinesterase inhibitor. Evid Based Complement Alternat Med 2016;2016.
- Zhao TY, Liu ZQ, Ma SF, Guo FF, Duan MH. Biatractylolide reduced amyloid beta protein-induced memory impairment in rats. Pak J Zool 2020;52(3):1031-8.
- Cao Q, Tan CC, Xu W, Hu H, Cao XP, Dong Q, et al. The prevalence of dementia: A systematic review and meta-analysis. J Alzheimers Dis 2020;73(3):1157-66.
[Crossref] [Google Scholar] [PubMed]
- Chenze JI, Shouchao WE, Tingting LI, Xiao BA, Wenrong CH, Zhimin LI, et al. The prevalence of vascular dementia in China: A systematic review and meta-analysis from 2009–2019. Iran J Public Health 2021;50(1):11-23.
[Crossref] [Google Scholar] [PubMed]
- Kuang H, Zhou ZF, Zhu YG, Wan ZK, Yang MW, Hong FF, et al. Pharmacological treatment of vascular dementia: a molecular mechanism perspective. Aging Dis 2021;12(1):308-26.
- Kim MS, Bang JH, Lee J, Han JS, Kang HW, Jeon WK. Fructus mume ethanol extract prevents inflammation and normalizes the septohippocampal cholinergic system in a rat model of chronic cerebral hypoperfusion. J Med Food 2016;19(2):196-204.
[Crossref] [Google Scholar] [PubMed]
- Iadecola C, Duering M, Hachinski V, Joutel A, Pendlebury ST, Schneider JA, et al. Vascular cognitive impairment and dementia: JACC scientific expert panel. J Am Coll Cardiol 2019;73(25):3326-44.
[Crossref] [Google Scholar] [PubMed]
- Anfinogenova ND, Quinn MT, Schepetkin IA, Atochin DN. Alarmins and c-Jun N-terminal kinase (JNK) signaling in neuroinflammation. Cells 2020;9(11):2350.
[Crossref] [Google Scholar] [PubMed]
- Zheng J, Dai Q, Han K, Hong W, Jia D, Mo Y, et al. JNK-IN-8, ac-Jun N-terminal kinase inhibitor, improves functional recovery through suppressing neuroinflammation in ischemic stroke. J Cell Physiol 2020;235(3):2792-9.
[Crossref] [Google Scholar] [PubMed]
- Schmidt J, Barthel K, Wrede A, Salajegheh M, Bähr M, Dalakas MC. Interrelation of inflammation and APP in sIBM: IL-1β induces accumulation of β-amyloid in skeletal muscle. Brain 2008;131(5):1228-40.
[Crossref] [Google Scholar] [PubMed]
- Schmitt TL, Steiner E, Klinger P, Sztankay A, Grubeck-Loebenstein B. The production of an amyloidogenic metabolite of the Alzheimer amyloid beta precursor protein (APP) in thyroid cells is stimulated by interleukin 1 beta, but inhibited by interferon gamma. J Clin Endocrinol Metab 1996;81(4):1666-9.
[Crossref] [Google Scholar] [PubMed]
- Grilli M, Goffi F, Memo M, Spano P. Interleukin-1 beta and glutamate activate the NF-kappaB/Rel binding site from the regulatory region of the amyloid precursor protein gene in primary neuronal cultures. J Biol Chem 1996;271(25):15002-7.
- Liao YF, Wang BJ, Cheng HT, Kuo LH, Wolfe MS. Tumor necrosis factor-alpha, interleukin-1 beta and interferon-gamma stimulate gamma-secretase-mediated cleavage of amyloid precursor proteinthrough a JNK-dependent MAPK pathway. J Biol Chem 2004;279(47):49523-32.
- Ledo JH, Liebmann T, Zhang R, Chang JC, Azevedo EP, Wong E, et al. Presenilin 1 phosphorylation regulates amyloid-β degradation by microglia. Mol Psychiatry 2020:1-6.
- Qin J, Zhang X, Wang Z, Li J, Zhang Z, Gao L, et al. Presenilin 2 deficiency facilitates Aβ-induced neuroinflammation and injury by upregulating P2X7 expression. Sci China Life Sci 2017;60(2):189-201.
[Crossref] [Google Scholar] [PubMed]
- Sheng JG, Bora SH, Xu G, Borchelt DR, Price DL, Koliatsos VE. Lipopolysaccharide-induced-neuroinflammation increases intracellular accumulation of amyloid precursor protein and amyloid β peptide in APPswe transgenic mice. Neurobiol Dis 2003;14(1):133-45.
[Crossref] [Google Scholar] [PubMed]
- Wu J, Mei C, Vlassara H, Striker GE, Zheng F. Oxidative stress-induced JNK activation contributes to proinflammatory phenotype of aging diabetic mesangial cells. Am J Physiol Renal Physiol 2009;297(6):F1622-31.
[Crossref] [Google Scholar] [PubMed]
- Borsello T, Forloni G. JNK signalling: A possible target to prevent neurodegeneration. Curr Pharm Des 2007;13(18):1875-86.
[Crossref] [Google Scholar] [PubMed]
- Moon J, Ha J, Park SH. Identification of PTPN1 as a novel negative regulator of the JNK MAPK pathway using a synthetic screening for pathway-specific phosphatases. Sci Rep 2017;7(1):1-9.
- Gao Y, Tu D, Yang R, Chu CH, Hong JS, Gao HM. Through reducing ROS production, IL-10 suppresses caspase-1-dependent IL-1β maturation, thereby preventing chronic neuroinflammation and neurodegeneration. Int J Mol Sci 2020;21(2):465.
[Crossref] [Google Scholar] [PubMed]
- Josef Schiefecker A, Dietmann A, Beer R, Pfausler B, Lackner P, Kofler M, et al. Neuroinflammation is associated with brain extracellular TAU-protein release after spontaneous subarachnoid hemorrhage. Curr Drug Targets 2017;18(12):1408-16.
[Crossref] [Google Scholar] [PubMed]
- Helbok R, Schiefecker A, Delazer M, Beer R, Bodner T, Pfausler B, et al. Cerebral tau is elevated after aneurysmal subarachnoid haemorrhage and associated with brain metabolic distress and poor functional and cognitive long-term outcome. J Neurol Neurosurg Psychiatry 2015;86(1):79-86.
[Crossref] [Google Scholar] [PubMed]
- Zanier ER, Zoerle T, Fiorini M, Longhi L, Cracco L, Bersano A, et al. Heart-fatty acid-binding and tau proteins relate to brain injury severity and long-term outcome in subarachnoid haemorrhage patients. Br J Anaesth 2013;111(3):424-32.
[Crossref] [Google Scholar] [PubMed]
- Su L, Surendranathan A, Huang Y, Bevan-Jones WR, Passamonti L, Hong YT, et al. Relationship between tau, neuroinflammation and atrophy in Alzheimer's disease: The NIMROD study. Inf Fusion 2021;67:116-24.
- Didonna A. Tau at the interface between neurodegeneration and neuroinflammation. Genes Immun 2020;21(5):288-300.
[Crossref] [Google Scholar] [PubMed]
- Hellmann J, Tang Y, Zhang MJ, Hai T, Bhatnagar A, Srivastava S, et al. Atf3 negatively regulates Ptgs2/Cox2 expression during acute inflammation. Prostaglandins Other Lipid Mediat 2015;116:49-56.
- Dusuel A, Deckert V, de Barros JP, van Dongen K, Choubley H, Charron E, et al. Human cholesteryl ester transfer protein lacks lipopolysaccharide transfer activity, but worsens inflammation and sepsis outcomes in mice. J Lipid Res 2021;62:100011.
[Crossref] [Google Scholar] [PubMed]
- Lee Y, Park Y, Nam H, Lee JW, Yu SW. Translocator protein (TSPO): The new story of the old protein in neuroinflammation. BMB Rep 2020;53(1):20.
[Crossref] [Google Scholar] [PubMed]
- She H, He Y, Zhao Y, Mao Z. Release the autophage brake on inflammation: The MAPK14/p38α-ULK1 pedal. Autophagy 2018;14(6):1097-8.
[Crossref] [Google Scholar] [PubMed]
- Hu L, Sun Y, Hu J. Catalpol inhibits apoptosis in hydrogen peroxide-induced endothelium by activating the PI3K/Akt signaling pathway and modulating expression of Bcl-2 and Bax. Eur J Pharmacol 2010;628(1-3):155-63.
[Crossref] [Google Scholar] [PubMed]