- *Corresponding Author:
- K. N. Varalakshmi
Department of Biotechnology, Center for Post Graduate Studies, Jain University, Bangalore 560 011 India
E-mail: kn.varalakshmi@jainuniversity.ac.in
| Date of Submission | 06 June 2016 |
| Date of Revision | 26 September 2016 |
| Date of Acceptance | 01 November 2016 |
| Indian J Pharm Sci 2016;78(6):725-731 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The aim of the current study was to evaluate the anticancer potential of tamarind bark and its bioactive fraction on HeLa and PA-1 cell lines. Tamarind bark extract was prepared with dichloromethane as the solvent using Soxhlet apparatus and treated on HeLa and PA-1 cell lines to determine the anticancer potential by 3-(4, 5-dimethyl thiazol-2-yl)-2, 5-diphenyl tetrazolium bromide assay. Further, bioassay guided fractionation of the extract was carried out using thin layer chromatography. In vitro assays like fluorescence microscopy, DNA fragmentation analysis, caspase-9 activity assay, and flow cytometry analyses were carried out to determine the mechanism of anticancer activity of the selected bioactive fraction. The fraction was further characterized by gas chromatography–mass spectrometry analysis. Tamarind bark dichloromethane extract was found to be cytotoxic to HeLa and PA-1 cells. Bioassay guided fractionation indicated that the fourth fraction of the extract was the bioactive component. Further in vitro assays demonstrated that the bioactive fraction was inducing caspase-9 mediated apoptosis in the cells and was able to reduce the total cell count of the cells as evidenced by flow cytometry analyses. The gas chromatography–mass spectrometry analysis of the bioactive fraction has suggested the presence of cantharidin, an anticancer compound earlier reported from the blister beetles. It can be concluded that the presence of cantharidin might be responsible for inhibition of proliferation and induction of apoptosis in the cancer cells.
Keywords
Apoptosis, anticancer, cantharidin, caspase, tamarind bark extract
Cancer is one of the most dreaded diseases as mortality associated is very high despite the availability of modern treatment modalities[1,2]. Hence, research towards novel, more effective and tumor-specific treatment has gained momentum during the past decades. In this pursuit, several medicinal and dietary plants have provided many anticancer compounds till date[3,4]. Tamarindus indica L. commonly known as tamarind is one of the most important dietary plants in which all parts have some nutritional or medicinal value. It has a history that dates back to ancient tribal people who used to prepare decoctions of the parts and use it for medication. It was reported that tamarind bark and leaves were used to treat wounds in West and East Africa[5]. Fruit and leaf have laxative property, paste of dried seeds were used to set fractured bones, and decoction of the bark and leaves were used to treat jaundice[6-8]. The bark of tamarind tree was reported as to have antiinflammatory, analgesic and wound healing properties[9,10]. In West Africa, the macerated fresh bark of the young twigs was used both as a purgative and to relieve abdominal pain[11].
Cantharidin is a colorless, odourless terpene secreted by many species of blister beetles. Cantharidin, isolated from the Chinese blister beetles, Mylabris phalerata or M. cichorii, displays antitumor activity and induces apoptosis in many types of tumor cells. It can be poisonous to humans if taken internally. Externally it is a potent vesicant. Properly dosed and applied, the same properties have been used for effective topical medications for some conditions, such as treating patients with infection of the skin. It has been used as an anticancer agent by the Chinese for the treatment of hepatoma and esophageal carcinoma since a long time[12]. Numerous other studies have also shown that cantharidin has induced cytotoxic effects on cancer cells[13]. According to reports from several epidemiologic studies, chronic inflammatory diseases are usually associated with increased cancer risk[14,15]. Due to the existence of causal relationship between inflammation and cancer and the reported antiinflammatory property of tamarind bark, in the current study we aimed to analyse the anticancer potential of tamarind bark, also to identify the biochemical mechanism and the active component responsible for this activity.
Materials and Methods
Cell lines
HeLa and PA-1 cell lines were procured from National Centre for Cell Sciences (NCCS), Pune. HeLa cells were grown in Dulbecco’s modified eagles medium (HiMedia Laboratories Pvt Ltd) and PA-1 cell lines were grown in minimum essential medium containing 10% fetal bovine serum (HiMedia Laboratories Pvt Ltd). Cells were maintained below passage 20 and used in experiments during the linear phase of growth. Both the cell lines were used for the cytotoxicity tests.
Preparation of the crude extract and screening for cytotoxicity
Tamarind bark was collected from Indian Institute of Science (IISc), Bengaluru, India and dried under the shade. The dried bark was ground to a fine powder and 50 g of the powder was packed in a Whatman filter paper and extracted in a Soxhlet apparatus, using the solvent dichloromethane[16]. The extract was concentrated using a rotary evaporator, dried and stored at 4° till further use. Stock solution of 1 mg/ml of the extract was prepared with dimethyl sulphoxide (DMSO) and later diluted to desired concentrations using phosphate buffered saline (PBS).
MTT [3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide] assay
MTT assay was performed to determine the cytotoxic effect of tamarind bark extract on cancer cell lines[17]. Approximately 2×104 cells/well were seeded in 96 well microplates and incubated at 37° for 24 h. Different concentrations of the sample were added and the microplates were further incubated for 24, 48 and 72 h. After specified period of incubation, 20 μl of MTT was added and incubated in the dark for 3 h. After 3 h, 200 μl of DMSO was added to each well and the absorbance was recorded at 540 nm in an enzyme linked immune sorbent assay (ELISA) plate reader (LISA Plus). Percent inhibition was calculated using the formula, Inhibition (%)=(1–ODs/OD)×100, where, ODs was the optical density of the sample and OD was the optical density of the control.
Bioassay guided fractionation by thin layer chromatography (TLC)
To identify the bioactive fraction, TLC was used. TLC plates (TLC silica gel 60 F254) were procured from Merck Specialties Private Limited, Mumbai. Different combinations of solvents with different concentrations were tried and the best solvent combination was used for separating the fractions from tamarind bark extract[18]. The fractions were collected by preparative TLC, dissolved in methanol and were dried. The dried fractions were again tested for cytotoxicity by MTT assay and the fraction with highest cytotoxic property was selected as the bioactive fraction.
Fluorescence microscopy
HeLa and PA-1 cells were cultured in 25 cm2 culture flasks and treated with different concentrations of tamarind bark bioactive fraction for 24 h. A control was maintained simultaneously. After incubation for the indicated time period, the control and treated cells were harvested separately, washed with PBS. The cells were treated with acridine orange (100 μg/ml) and ethidium bromide (100 μg/ml) stain prepared in the ratio 1:1, mounted on slides and were observed under a fluorescence microscope[19].
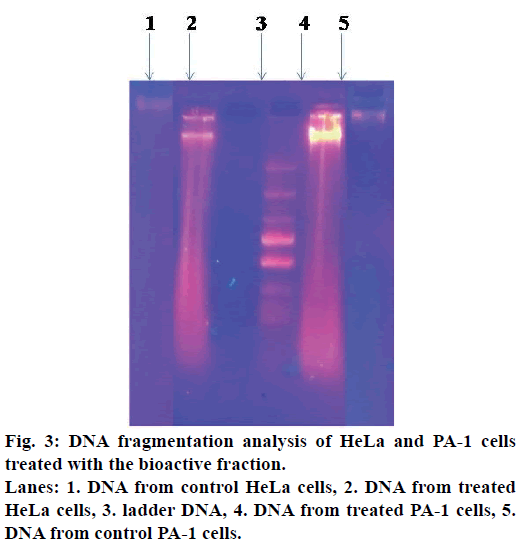
DNA fragmentation analysis
HeLa and PA-1 cells (2×104 cells/ml) were treated with different concentrations of tamarind bark bioactive fraction and incubated for 24 h. simultaneously, a control was maintained. Cells were washed with PBS, trypsinised, centrifuged and the cell pellet was lysed in buffer containing 10 mM Tris HCl, 10 mM EDTA, 0.5% Triton X-100. To avoid RNA and protein contamination, 200 μg/ml of RNase and 200 μg/ml proteinase K were added. DNA was precipitated with ice cold ethanol and suspended in Tris-EDTA solution. Samples were resolved using 0.8% agarose gel and visualized under a UV transilluminator[20].
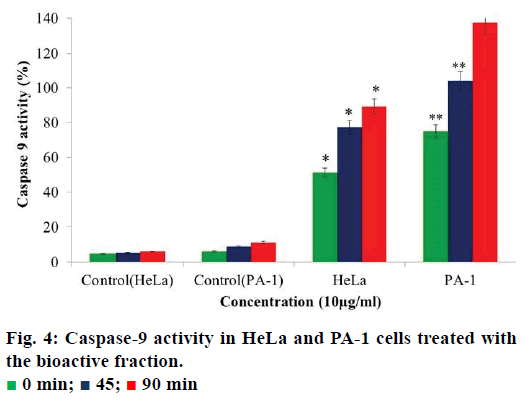
Caspase-9 activity assay
The assay was performed using caspase-9 apoptosis detection kit (G-Biosciences) according to the manufacturer’s instructions. Cells were treated with 5 μg/ml of tamarind bark bioactive fraction for 24 h and simultaneously a control was maintained. After 24 h, cells were collected by trypsinisation and treated with 50 μl of lysis buffer. The lysate was frozen and thawed 3 times for the even suspension of the cells in the buffer. The suspension was centrifuged and the supernatant was collected. Fifty microliters of caspase assay buffer containing 1 M DTT (dithiothreitol) was added to the lysate. A total of 5 μl of AFC conjugated substrate (50 μM final concentration) was added and incubated at 37° for 2 h. Absorbance was recorded every 15 min at 405 nm in an ELISA plate reader. Percent caspase activity was calculated using the following formula, OD control/sample-OD blank/OD blank×100
Annexin V apoptosis assay
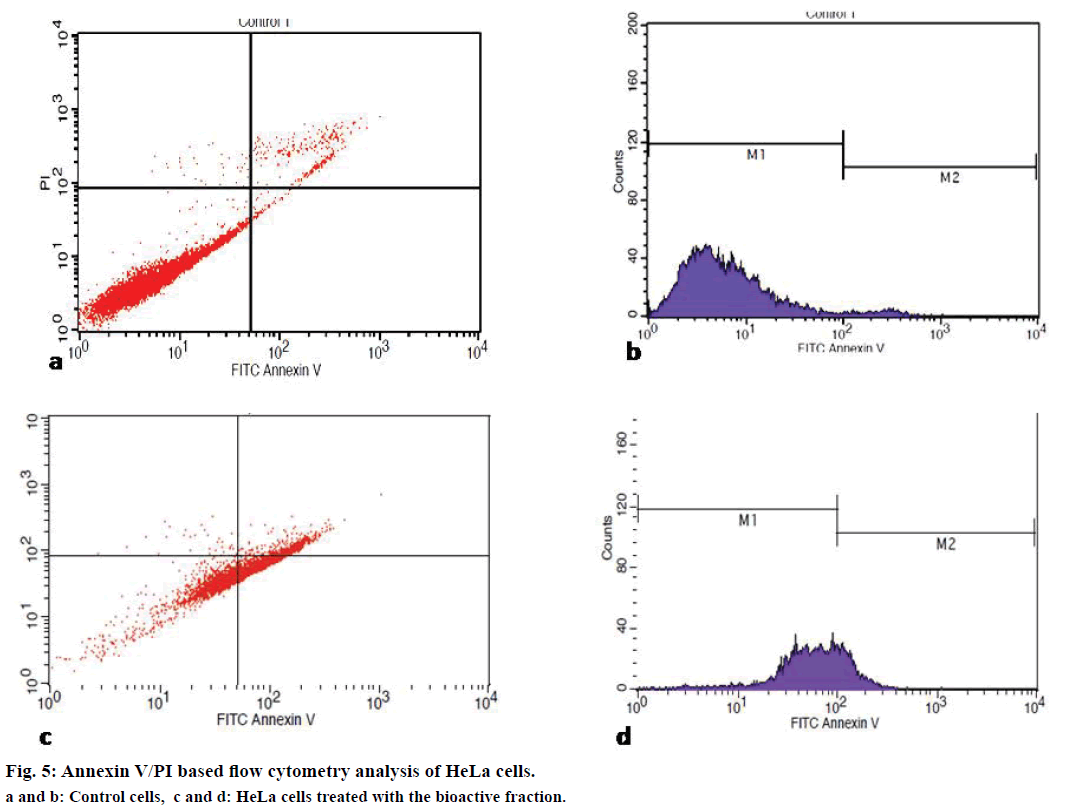
Annexin V coupled with propidium iodide (PI) staining is the most common approach used for the detection of apoptosis in a flow cytometer[21]. The cell population undergoing apoptosis, necrosis and also the population of viable cells can be analysed from this assay. HeLa cells at a concentration of 1×106 cells/ml were seeded in a culture flask and after 24 h treated with tamarind bark violet fraction and incubated further for 24 h. The cells later were harvested by trypsinisation, stained with Annexin V and PI and analysed with the help of a flow cytometer.
Gas chromatography-mass spectrometry (GC-MS) analysis
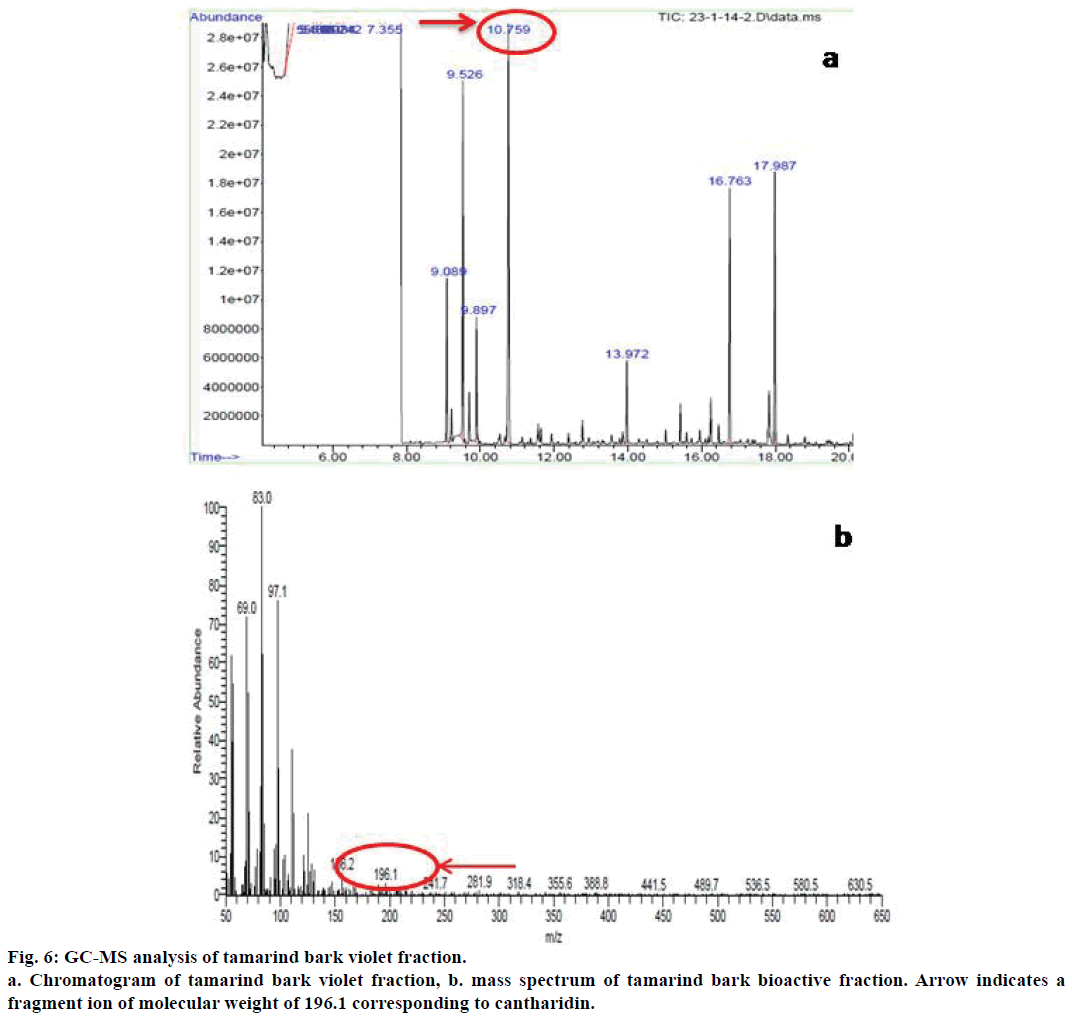
The bioactive fraction of tamarind bark was subjected to GC-MS analysis. For GC-MS analysis, Thermo GC-Trace Ultra version 5.0, equipped with Thermo MS DSQ II mass spectrometer were used. DB 5-MS Capillary Standard non polar column was used with dimensions 30×0.25 mm and film thickness 0.25 μm. Helium gas was used as a carrier gas with a flow rate of 1 ml/min. The oven temperature initially was 70° which was gradually increased to 260° at the rate of 6°/ min. 1 μl of the sample was injected to the column and the mass spectra were obtained within the mass range of 50-650 atomic mass unit (AMU). The mass spectra thus obtained were compared with the known mass spectra from NIST library and the molecular weight and structure of the compounds were determined[22].
Statistical analysis
All experiments were carried out in triplicates. The results were calculated as mean±standard error (SE) values. Statistical significance was calculated using one way analysis of variance (ANOVA) and Dunnett’s multiple comparison tests. The values P<0.05 were taken as significant.
Results and Discussion
The current study demonstrated the anticancer effects of tamarind bark, an important nutritive and medicinal plant. Several medicinal properties were reported for T. indica. It has broad spectrum of antibacterial activity. The methanol extract of the leaf of this plant was found to possess strong antibacterial activity[23]. The seed and pericarp of tamarind have been reported to contain phenolic antioxidant compounds. Extracts from all parts of T. indica exhibit good antioxidant activities[24]. Tamarind is often used for the treatment of cuts, wounds and abscesses. The bark or leaves are most commonly used, is applied externally on the spot, either as a decoction or as a powder or poultice, alone or in combination with other species[25]. T. indica bark has been traditionally used for the treatment of pain. Petroleum ether extract of the bark had significant analgesic activity in mice models. The presence of sterols and triterpenes were reported in the bark extract[26]. But to the best of our knowledge, anticancer activity of T. indica bark has not been reported in literature. Because of the causal relationship between inflammation and cancer and the reported antiinflammatory activity of tamarind bark, in the current study we tried to analyse the anticancer activity of tamarind bark extract.
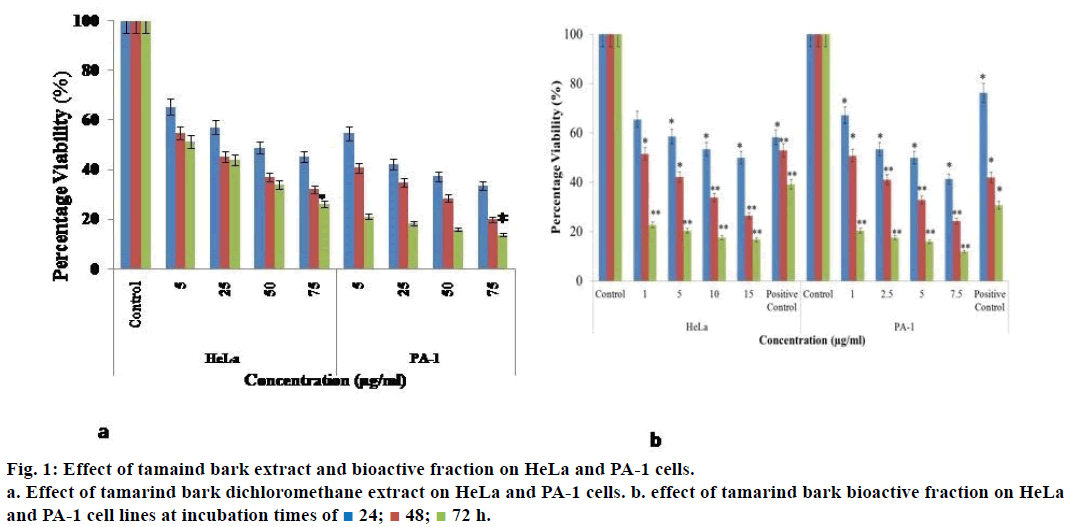
When HeLa cells were treated with 5 μg/ml of the dichloromethane extract of tamarind bark, percent cell viability decreased to 65.4% and as the concentration of the extract increased to 75 μg/ml and the % viability decreased further to 45.2% (Figure 1a). As the incubation of treatment was increased to 72 h, a drastic decrease in the percent viability of the cells was observed with a mere 26.3% at 75 μg/ml treatment. A similar effect was observed even in the case of PA-1 cells.
As the extract demonstrated promising results, we attempted to fractionate the components in the tamarind bark by TLC. The solvent combination of toluene:ethyl acetate (9:1) as the mobile phase, we could get 7 distinct fractions (results not shown). Among these the 4th fraction was cytotoxic to HeLa and PA-1 cells. When HeLa and PA-1 cells were treated with this bioactive fraction for 24, 48 and 72 h, there was a decrease in the percentage viability of the cells in a dose and time dependent manner (Figure 1b). From the dose response curve, the IC50 value of this bioactive fraction was calculated as 7.5 μg/ml on HeLa and 0.1 μg/ml on PA-1 cells. As this fraction had exhibited antiproliferative properties on both the cancer cell lines, we wanted to analyze its mechanism of action by observing the morphology of the cells through fluorescence microscopy. Here, we could notice nuclear disintegration of the treated cells as compared to the control cells. Viable cells with intact membrane were fluorescing bright green and treated cells were bright orange in colour (Figure 2). The results clearly suggested that the bioactive fraction had brought about apoptotic changes in the cells.
DNA fragmentation is one of the characteristics of apoptotic cells. The apoptogenic activity of the bioactive fraction was further supported from the DNA patterns after agarose gel electrophoresis. When observed under the UV transilluminator, DNA from the treated cells appeared as a smear, whereas the DNA from the control cells was an intact band (Figure 3).
Caspase-9 is an initiator caspase that activates all the procaspases thereby bringing about apoptosis in the cells. An increase in caspase-9 activity in cancer cells can be regarded as an indication of cells undergoing apoptosis. When we analysed the activity of caspase-9 in the cells treated with the bioactive fraction, it was observed that the caspase activity in HeLa cells the activity was 51.2% and in PA-1 cells it was 75% (Figure 4). The caspase-9 activity increased to 77.3% in HeLa cells and 104% in PA-1 cells after 45 min. When the time of incubation was increased to 90 min, the caspase-9 activity increased to 89.8% in HeLa cells and 137.5% in PA-1 cells. As compared to the control cells, the increase in caspase-9 activity was 83.7% in HeLa and 125.7% in PA- 1 cells. This gave further evidence for the induction of apoptosis in the treated HeLa and PA-1 cells by this fraction.
Irresponsive to cell cycle signals is one of the mechanisms employed by cancer cells while evading apoptosis and resulting in rapid cell proliferation. Therefore, quantitative analysis of cell cycle has become a major criterion for the determination of cell death. To determine the effect of the bioactive fraction on the different stages of cell cycle, we subjected the treated cancer cells to cell cycle analysis by flow cytometry after Annexin V/PI staining. We found that live cells were 17% in treated flasks as compared to the control flask where it was 95.9%. Apoptotic cells increased to 60% in the bioactive fraction treated flasks from 3% in the control flasks (Figure 5). Our cell cycle analysis results provided a strong evidence for the apoptotic induction of cell death by the bioactive fraction.
As the results of all these assays provided proof for the anticancer potential of the bioactive fraction from tamarind bark, we further proceeded to characterize the compound responsible for this bioactivity through GC-MS analysis. The GC-MS analysis of the bioactive fraction indicated the presence of a major peak at retention time 10.75 min (Figure 6a). The mass spectral analysis coupled with library search for this peak has resulted in a major fragment with 100% relative abundance having a mass to charge ratio (m/z) of 83 as the base peak (Figure 6b) and another fragment ion with a mass to charge ratio of 196.1. This value was corresponding to the molecular weight of cantharidin as per the library search results. Cantharidin was earlier reported for its anticancer activity from the blister beetle M. phalerat[27].
Hence, we assume that the presence of this compound might be responsible for the observed anticancer activity of tamarind bark bioactive fraction. To the best of our knowledge, this is the first report of cantharidin from tamarind bark, which possibly could be the contributing compound towards the anticancer activity observed from this plant source. We conclude by saying that cantharidin might be responsible for the antiproliferative and apoptogenic activity of tamarind bark and this common plant holds promise towards future cancer treatment strategies.
Acknowledgements
The authors are grateful to the management, Jain Group of Institutions for the infrastructural facilities provided.
Conflict of interest
There is no conflict of interest.
Financial support and sponsorship
The financial support provided in the form of junior research fellowship.
References
- Jeong CH, Bode AM, Pugliese A, Cho Y, Kim H, Shim J, et al.6-gingerol suppresses colon cancer growth by targeting leukotriene A4 hydrolase. Cancer Res 2009;69:5584-91.
- Garg M, Lata K, Satija S. Cytotoxic potential of few Indian fruit peels through 3-(4,5-dimethylthiazol-yl)-2,5-diphenyltetrazolium bromide assay on HepG2 cells. Indian J Pharmacol 2015;48:64-8.
- Patel B, Das S, Prakash R, Yasir M. Natural bioactive compound with anticancer potential. Int J Adv pharm Sci 2010;1:32-41.
- Sukhdev. Ancient modern concordance in ayurvedic plants: some examples. Environ Health Pers 107:783-9.
- Havinga RM, Hartl A, Putscher J, Prehsler S, Buchmann C,Vogl CR. Tamarindusindica L. (Fabaceae): Patterns of use in traditional African medicine. J Ethnopharmacol 2010;127:573-88.
- El-Siddig K, Gunasena,HPM, Prasa BA, Pushpakumara DKNG, Ramana KVR, Vijayanand P, et al.Tamarind-TamarindusindicaL. Fruits for the future. Southampton Centre for Underutilized Crops, Southampton 2006;188.
- Vaidyanathan D, SalaiSenthilkumar MS, GhouseBasha M. Studies on ethnomedicinal plants used by malayalitribals in Kolli hills of Eastern ghats, Tamilnadu, India. Asian J Plant Sci Res 2013;3:29-45.
- Caluwe ED, Halamova K, Damme PV. TamarindusindicaL. A review of traditioaluses,phytochemistry and pharmacology. Afrika Focus 2010;23:53-83.
- Irvine FR. Woody Plants of Ghana. Oxford University Press 1961.
- Morton J. Tamarind. In: Morton JF, editor. Fruits of warm climates.1987. p. 115-121.
- Bhat RB, Eterjere EO, Oladipo VT. Ethnobotanical studies from Central Nigeria. Econ Bot 1990;44:382-90.
- Liu D, Chen Z. The effects of cantharidin and cantharidinderivates on tumour cells. Anticancer Agents Med Chem 2009;9:392-6.
- Hsia TC, Yu CC, Hsu SC, Tang NY, Lu HF, Huang YP, et al. Cantharidin induces apoptosis of H460 human lung cancer cells through mitochondria-dependent pathways. Int J Oncol 2014;45:245-54.
- Lee YJ, Shacter E. Oxidative stress inhibits apoptosis in human lymphoma cells. J BiolChem 1999;9:19792-8.
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860-7.
- Hayashi Y, Nishikawa Y, Mori H, Tamura H, Matsushita Y, Matsu T. Antitumor activity of (1 OE, 122)-9-hydroxy-10,12-Octadecadienoic acid from rice bran. J Ferment Bioeng1998;86:149-53.
- Mosmann T. Rapid calorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55-63.
- Cieśla ŁM, Waksmundzka-Hajnos M. Application of thin layer chromatography for the quality control and screening the free radical scavenging activity of selected pharmaceutical preparations containing SalviaofficinalisL. extract. Acta Pol Pharm 2010;67:484-5.
- Roy S, Besra SE, De T, Banerjee B, Mukherjee J, Vedasiromoni JR, et al. Induction of apoptosis in human leukemic cell lines U937, K562 and HL-60 by Litchi chinensisleaf extract viaactivation of mitochondria mediated caspase cascades. Open Leuk J 2008;1:1-14.
- Lee YJ, Shacter E. Oxidative stress inhibits apoptosis in human lymphoma cells. J BiolChem1999;9:19792-8.
- Dicker DT, Kim SH, Jin Z, El-Deiry WS. Heterogeneity in non-invasive detection of apoptosis among human tumor cell lines using annexin-V tagged with EGFP or Qdot-705. Cancer BiolTher2005;4:1014-17.
- Belayachi L, Aceves-Luquero C, Merghoub N, Bakri Y, Fernández de Mattos S, Amzazi S, et al. Retamamonosperman-hexane extract induces cell cycle arrest and extrinsic pathway-dependent apoptosis in Jurkat cells. BMC ComplemAltern Medicine 2014;14:1-12.
- Meléndez PA, Capriles VA. Antibacterial properties of tropical plants from Puerto Rico.Phytomedicine 2006;13:272-6.
- Siddhuraju P. Antioxidant activity of polyphenolic compounds extracted from defatted raw and dry heated Tamarindusindica seed coat. LWT Food SciTechol2007;40:982-90.
- Diallo D, Sogn C, Samaké FB, Paulsen BS, Michaelsen TE, Keita A. Wound healing plants in Mali, the Bamako region. An ethnobotanical survey and complement fixation of water extracts from selected plants. Pharm Biol 2002;40:117-28.
- Dighe NS, Pattan SR, Nirmal SA, Kalkotwar RS, Gaware VM, Hole MB. Analgesic activity of Tamarindusindica. Res J PharmacognPhytochem2009;1:69-71.
- Sakoff JA, Ackland SP, Baldwin ML, Kaene MA, McClusky A. Anticancer activity and protein phosphatase 1 and 2a inhibition of a new generation of cantharidin analogues. Invest New Drugs 2002;20:1-11.
 24;
24;  48;
48;  72 h.
72 h.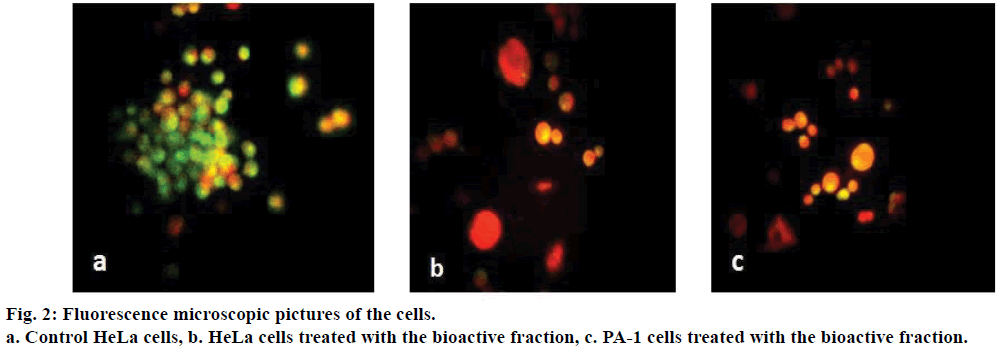
 0 min;
0 min;  45;
45;  90 min
90 min