- *Corresponding Author:
- H. Qian
Department of Medical Oncology, The People’s Liberation Army (PLA) Navy Anqing Hospital, Anqing, Anhui Province 246003, China
E-mail: qh875623@163.com
| This article was originally published in a special issue, “Recent Developments in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(4) Spl Issue “25-30” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To clarify association of epidermal growth factor receptor, human epidermal growth factor receptor 2 with prognosis of colorectal cancer under FOLFOX chemotherapy. A total of 108 colorectal cancer patients under FOLFOX chemotherapy in the hospital from October 2017 to October 2019 were chosen as research subjects. The baseline data of patients were recorded before treatment and levels of serum tumor markers carcino embryonic antigen and carbohydrate antigen 125 and serum epidermal growth factor receptor and human epidermal growth factor receptor 2 were measured and recorded. After 6 courses of FOLFOX chemotherapy, prognosis of patients was observed. The effective patients were included in good prognosis group and the remaining ones were included in poor prognosis group. The baseline data and laboratory indicators before treatment were assessed between two groups and association of epidermal growth factor receptor and human epidermal growth factor receptor 2 with prognosis of colorectal cancer under FOLFOX chemotherapy was evaluated. Among 108 colorectal cancer patients completing FOLFOX chemotherapy, 18 (16.67 %) had complete response, 29 (26.85 %) had partial response, 37 (34.26 %) had stable disease and 24 (22.22 %) had progressive disease and 56.48 % (61/108) had poor prognosis. Carcino embryonic antigen, carbohydrate antigen 125, epidermal growth factor receptor and human epidermal growth factor receptor 2 in poor prognosis group presented elevation relative to those in good prognosis group, with statistical significance (p<0.05). Logistic regression analysis demonstrated that carcino embryonic antigen, carbohydrate antigen 125, epidermal growth factor receptor and human epidermal growth factor receptor 2 had association with prognosis of colorectal cancer under FOLFOX chemotherapy (odds ratio >1, p<0.05). The receiver operating curve demonstrated that area under curve of serum epidermal growth factor receptor and human epidermal growth factor receptor 2 levels before treatment alone and in combination to predict prognosis of colorectal cancer under FOLFOX chemotherapy were 0.709, 0.766 and 0.828, respectively, all of which had certain predictive value. The up regulation of serum epidermal growth factor receptor and human epidermal growth factor receptor 2 may be a risk factor for unfavourable prognosis of colorectal cancer under FOLFOX chemotherapy, which can be used for predicting prognosis of colorectal cancer under FOLFOX chemotherapy.
Keywords
Colorectal cancer, FOLFOX, epidermal growth factor receptor, human epidermal growth factor receptor 2
Colorectal Cancer (CRC), with high mortality, ranks fifth globally. Nevertheless, CRC patients are generally diagnosed late and most patients have distant metastasis or advanced stages when they are diagnosed, not suitable for surgical therapy[1]. FOLFOX chemotherapy based on fluorouracil and with a high objective response rate, is the most widely applied chemotherapy regimen for CRC[2]. It makes optimization of FOLFOX chemotherapy a research hotspot in therapy for CRC and it is essential to evaluate and predict patients before treatment. Nevertheless, chemotherapy efficacy is regulated by many factors, and it is difficult to predict prognosis of FOLFOX chemotherapy. Epidermal Growth Factor Receptor (EGFR) and Human Epidermal Growth Factor Receptor 2 (HER2) are the most researched markers in recent years. Among them, EGFR is a trans membrane glycoprotein with cytoplasmic tyrosine kinase activity, which may get involvement in tumor survival, invasion, metastasis, angiogenesis and other pathogenic mechanisms[3]. At present, there are multiple reports related to EGFR. EGFR affects triple-negative breast cancer tumor cell proliferation, invasion and migration and has relation to tumor prognosis[4]. HER2 has biological functions such as regulating cell proliferation, differentiation, apoptosis, etc., exerting a crucial role in occurrence and development of multiple malignancies[5]. HER2 has certain value as a target for breast cancer diagnosis and therapy in clinical breast cancer patients[6]. Nevertheless, there are few relevant reports on whether EGFR and HER2 can be applied to predict prognosis of CRC under FOLFOX chemotherapy. Based on this, our research evaluated association of EGFR and HER2 with prognosis of CRC under FOLFOX chemotherapy, providing a reference for optimization of subsequent CRC therapeutic plans.
Materials and Methods
General data:
A total of 108 CRC patients receiving FOLFOX chemotherapy in the hospital from October 2017 to October 2019 were chosen as research subjects, including 61 males and 47 females, aged 36 y-69 y old, mean (57.00±6.26) y old; Body Mass Index (BMI) 18.72-24.97 kg/m2, mean (22.41±0.85) kg/m2; tumor location 44 cases of colon and 64 cases of rectum; Tumor, Nodes and Metastases (TNM) stage[7] 37 cases of stage III and 71 cases of stage IV.
Inclusion and exclusion criteria:
Inclusion criteria: All met the diagnostic criteria in the clinical practice guidelines for colon and rectal cancers 2017 edition published online by the National Comprehensive Cancer Network[8]; confirmed as CRC by endoscopy; solitary; Karnofsky Performance Scale (KPS)[9] score ≥70 points and eligible for chemotherapy.
Exclusion criteria: Recurrence; severe liver injury; complicated with infectious diseases; intolerance to FOLFOX chemotherapy; allergy to study drugs and had received relevant therapy before enrollment.
Methods:
Data collection methods and laboratory indicator detection methods: The baseline data of patients were collected, including gender, age, TNM stage, tumor diameter, tumor location, etc. Before treatment, 2 ml of fasting venous blood was collected and centrifuged at 4000 r/min for 10 min. A Chemiluminescence Immunoassay (CLIA)analyzer (Abbott AXSYM, USA) determined serum Carcino Embryonic Antigen (CEA) and Carbohydrate Antigen (CA) 125 levels through CLIA. Before treatment, 2 ml of fasting venous blood was collected and centrifuged at 3000 r/min for 12 min. The kits provided by Shanghai Enzyme Linked Bio detected serum EGFR and HER2 through Enzyme-Linked Immunosorbent Assay (ELISA).
Treatment methods: FOLFOX chemotherapy was implemented, 4 w as a course of treatment, a total of 6 courses of treatment. On the 1st d of each course of treatment, 100 mg/m2 oxaliplatin (G. Y. Z. Zi. H20123347, Shandong Luoxin Pharmaceutical Group Stock co., ltd.); specification 100 mg was intravenously infused for 2 h. On the 1st d-5th d, 200 mg/m2 tetrahydrofolic acid (G. Y. Z. Zi. H20113120, Youcare Pharmaceutical Group; specification: 100 mg) and 500 mg/m2 5-fluorouracil (G. Y. Z. Zi. H31020593, Shanghai Xudong Haipu Pharmaceutical Co., Ltd.; specification: 0.25 g) were intravenously infused.
Prognosis assessment methods and grouping methods: After completion of FOLFOX chemotherapy, efficacy was evaluated according to the Response Evaluation Criteria In Solid Tumors (RECIST) Guidelines (Version 1.1)[10]. Tumor tissue completely disappeared and lasted for over 1 mo, which was a Complete Response (CR); product of tumor tissue maximum diameter and vertical diameter decreased by over 50 %, without new lesions and lasted for over 1 mo, which was a Partial Response (PR); product of two diameters decreased by <50 % but new lesions were <25 % and lasted for over 1 mo, which was a Stable Disease (SD); product of two diameters of new lesions was >25 %, which was a Progressive Disease (PD); total effectiveness=CR+PR. The effective patients were included in good prognosis group and the remaining ones were included in poor prognosis group.
Statistical analysis:
Statistical Package for Social Sciences (SPSS) 24.0 software was used for data processing. Measurement data were tested through Shapiro-Wilk normality test, exhibited by normal distribution and expressed as, using independent samples t test between groups. Enumeration data were expressed as percentages using the χ2 test and rank data using rank sum test. Logistic regression evaluated association of EGFR and HER2 with prognosis of CRC under FOLFOX chemotherapy. The Receiver Operating Curve (ROC) was drawn to obtain Area Under Curve (AUC) and value of serum EGFR and HER2 levels before treatment for predicting risk of poor prognosis of CRC under FOLFOX chemotherapy was assessed. AUC value >0.9 meant high predictive performance, 0.71-0.90 meant certain predictive performance and 0.5-0.7 meant poor predictive performance. p<0.05 meant that difference was statistically significant.
Results and Discussion
CEA, Cancer Antigen 125 (CA-125), EGFR and HER2 in poor prognosis group presented elevation relative to those in good prognosis group, with statistical significance (p<0.05, Table 1).
| Factors | - | Poor prognosis group (n=61) | Good prognosis group (n=47) | Statistics | p |
|---|---|---|---|---|---|
| Gender [n (%)] | Male | 36 (59.02) | 25 (53.19) | χ2=0.366 | 0.545 |
| Female | 25 (40.98) | 22 (46.81) | |||
| Age (x?±s, years) | 57.13±6.42 | 56.83±6.10 | t=0.247 | 0.805 | |
| TNM [n (%)] | III | 20 (32.79) | 17 (36.17) | Z=0.366 | 0.715 |
| IV | 41 (67.21) | 30 (63.83) | |||
| Tumor diameter (x?±s, cm) | 5.52±0.63 | 5.37±0.60 | t=1.324 | 0.188 | |
| Tumor location [n (%)] | Colon | 23 (37.70) | 21 (44.68) | χ2=0.535 | 0.465 |
| Rectum | 38 (62.30) | 26 (55.32) | |||
| CEA (x?±s, ng/ml) | 33.54±3.25 | 29.58±3.06 | t=6.421 | <0.001 | |
| CA125 (x?±s, IU/ml) | 5.75±0.61 | 5.13±0.55 | t=5.508 | <0.001 | |
| EGFR (x?±s, mg/l) | 7.54±0.85 | 6.95±0.72 | t=3.835 | <0.001 | |
| HER2 (x?±s, mg/l) | 9.78±1.13 | 8.66±0.91 | t=5.553 | <0.001 |
Table 1: Baseline Data and Laboratory Indicators in two Groups.
The variables with statistical significance in comparison results were included as independent variables and prognosis of CRC under FOLFOX chemotherapy was used as dependent variable (1=poor prognosis, 0=good prognosis). CA125, EGFR and HER2 had association with prognosis of CRC under FOLFOX chemotherapy (Odd Ratio (OR) >1, p<0.05, Table 2).
| Factors | B | SE | Wald | p | OR | 95 % CI |
|---|---|---|---|---|---|---|
| Constant | -46.724 | 9.83 | 22.595 | <0.001 | - | - |
| CEA | 0.534 | 0.145 | 13.632 | <0.001 | 1.706 | 1.285-2.266 |
| CA125 | 2.281 | 0.621 | 13.511 | <0.001 | 9.791 | 2.901-33.048 |
| EGFR | 1.196 | 0.469 | 6.507 | 0.011 | 3.306 | 1.319-8.286 |
| HER2 | 1.027 | 0.323 | 10.084 | 0.001 | 2.792 | 1.481-5.261 |
Table 2: The Association of EGFR and HER2 with prognosis of CRC under FOLFOX Chemotherapy.
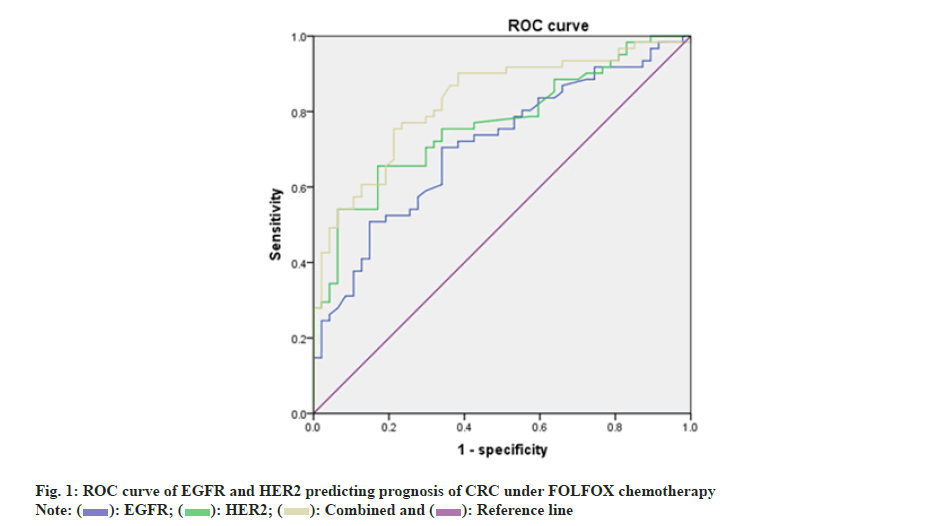
Taking prognosis of CRC under FOLFOX chemotherapy as a status variable (1=poor prognosis, 0=good prognosis) and taking serum EGFR and HER2 levels before treatment as test variables, ROC curve was drawn as shown in fig. 1. The AUCs of EGFR and HER2 levels alone and in combination to predict prognosis of CRC under FOLFOX chemotherapy were 0.709, 0.766 and 0.828, respectively, all of which had certain predictive value as shown in Table 3.
| Factors | AUC | Cut-off value | 95 % CI | p | Specificity | Sensitivity | Youden index |
|---|---|---|---|---|---|---|---|
| EGFR | 0.709 | 7.1 | 0.613-0.806 | <0.001 | 0.66 | 0.705 | 0.365 |
| HER2 | 0.766 | 8.845 | 0.678-0.855 | <0.001 | 0.681 | 0.721 | 0.402 |
| Combination | 0.828 | - | 0.751-0.905 | <0.001 | 0.787 | 0.754 | 0.541 |
Table 3: Serum EGFR and HER2 before treatment predicted prognosis of CRC under FOLFOX Chemotherapy.
CRC, the third largest malignancy in the world, with high morbidity and mortality, is an urgent problem to be solved[11]. Chemotherapy is one of the main therapy methods for patients. FOLFOX is a widely applied chemotherapy regimen in CRC patients, which can effectively kill tumor cells and has a high therapeutic efficacy[12]. Thus, how to better predict prognosis of CRC patients under FOLFOX chemotherapy exerts a crucial role, beneficial to guide adjustment of program and improve prognosis.
Herein, among 108 CRC patients completing FOLFOX chemotherapy, 56.48 % had unfavorable prognosis. It suggests that prognosis of CRC after FOLFOX chemotherapy still needs to be improved and it is necessary to analyze related prognostic factors after FOLFOX chemotherapy. EGFR is a multifunctional glycoprotein, which is distributed in cell membranes of various tissues of human body. It can promote survival, differentiation, adhesion and movement of cancer cells under mediation of signal transduction pathways and can also repair damaged cancer cells, which may have relation to treatment efficacy and prognosis of CRC patients[13]. EGFR activation gets involvement in signal transduction pathways regulating cell proliferation, differentiation and survival, is overexpressed in a variety of epithelial-derived tumors, and has association with unfavorable prognosis of cancer patients[14]. HER2 is a proto-oncogene, a member of Erythroblastic Leukemia Viral Oncogene Homologue (ERBB) family. The protein encoded by HER2 has a special structure, that is, it does not bind ligands, but can form heterodimers through binding to other ERBB family members, stimulating enhancement of tyrosine kinase activity and activating downstream signaling pathways, which may have relation to tumor occurrence and development[15]. The rate of microsatellite unstable mutation in advanced breast cancer with high HER2 expression is also quite high, and loss of microsatellite heterozygosity may have relation to angiogenesis[16]. Nevertheless, association of EGFR and HER2 with CRC also lacks a relatively unified opinion, thus further research is needed.
Herein, EGFR and HER2 in poor prognosis group presented elevation relative to those in good prognosis group. EGFR and HER2 had relation to prognosis of CRC under FOLFOX chemotherapy. The reason may be that EGFR is widely distributed on cell membranes of various tissues. As a multifunctional glycoprotein, it can bind to corresponding ligands such as EGF, Transforming Growth Factor (TGF), etc., to form a dimer, which attaches to cell surface, activates protein kinases and transmits signals through phosphorylation via binding to its own ligands, mediating cell differentiation, survival, migration, invasion, adhesion, repair of cell damage, etc.[17]. High serum EGFR level means more EGFR activation, which facilitates rapid proliferation of malignant tumor cells, increases new blood vessels, accelerates tumor metastasis, suppresses apoptosis of cancer cells and aggravates degree of malignancy, which may affect prognosis of CRC patients[18]. EGFR can stimulate malignant tumor cell proliferation, which has relation to tumor progression and it exerts a vital role in malignant outcome of malignant tumor patients[19]. HER2 is a transmembrane glycoprotein with tyrosine kinase activity repressing cell apoptosis. It suppresses cancer cell apoptosis, accelerates cancer cell proliferation, facilitates new blood vessel formation and enhances cancer cell invasive ability, affecting prognosis of chemotherapy[20]. By combining the Ras/Mitogen-Activated Protein Kinase (MAPK) signaling pathway, HER2 can stimulate proliferation, differentiation and metastasis of cancer cells, and aggravate CRC progression[21]. HER2 is overexpressed in gastric cancer and has association with disease progression and prognosis[22].
Additionally, herein, ROC curve was drawn. AUCs of pre-treatment serum EGFR and HER2 levels alone and in combination to predict prognosis of CRC under FOLFOX chemotherapy were 0.709, 0.766 and 0.828, respectively, all of which had certain predictive value. Nevertheless, this research did not assess association of EGFR and HER2 with long-term prognosis of CRC under FOLFOX chemotherapy, which has certain limitations, and follow-up time should be extended to continue the research.
In conclusion, up-regulation of serum EGFR and HER2 may be a risk factor for unfavourable prognosis of CRC under FOLFOX chemotherapy, which can be used for predicting prognosis of CRC under FOLFOX chemotherapy.
Author’s contributions:
Hong Qian and Wenzhi Huang have contributed equally to this work.
Acknowledgement:
This work was supported by the PLA Navy Anqing Hospital.
Conflict of interests:
The authors declared no conflict of interests.
References
- Piawah S, Venook AP. Targeted therapy for colorectal cancer metastases: A review of current methods of molecularly targeted therapy and the use of tumor biomarkers in the treatment of metastatic colorectal cancer. Cancer 2019;125(23):4139-47.
[Crossref] [Google Scholar] [PubMed]
- Pietrantonio F, Fucà G, Rossini D, Schmoll HJ, Bendell JC, Morano F, et al. FOLFOXIRI-bevacizumab or FOLFOX-panitumumab in patients with left-sided RAS/BRAF wild-type metastatic colorectal cancer: A propensity score-based analysis. Oncologist 2021;26(4):302-9.
[Crossref] [Google Scholar] [PubMed]
- Zanwar S. Epidermal growth factor receptor mutated lung cancers: Looking beyond adenocarcinomas. Indian J Cancer 2021;58(1):3-4.
- Zhang H, Zheng Y, Zhang Y. Knockdown of TRIM66 in MDA-MB-468 triple negative breast cancer cell line suppresses proliferation and promotes apoptosis through EGFR signaling. Pol J Pathol 2021;72(2):160-6.
[Google Scholar] [PubMed]
- Nagaraj G, Ma CX. Clinical challenges in the management of hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer: A literature review. Adv Ther 2021;38(1):109-36.
[Crossref] [Google Scholar] [PubMed]
- Rajarajan N, Mariotti V, Basu A, Kodumudi K, Han H, Czerniecki B, et al. Strategies to combat human epidermal growth factor receptor 2 (HER2) resistance in HER2-positive breast cancer. Crit Rev Oncog 2020;25(3):209-31.
[Crossref] [Google Scholar] [PubMed]
- Shida D, Kanemitsu Y, Hamaguchi T, Shimada Y. Introducing the eighth edition of the tumor-node-metastasis classification as relevant to colorectal cancer, anal cancer and appendiceal cancer: A comparison study with the seventh edition of the tumor-node-metastasis and the Japanese classification of colorectal, cppendiceal and anal carcinoma. Jpn J Clin Oncol 2019;49(4):321-8.
[Crossref] [Google Scholar] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology. Colon Cancer. Version I; 2017.
- Çeltek NY, Süren M, Demir O, Okan ?. Karnofsky Performance Scale validity and reliability of Turkish palliative cancer patients. Turk J Med Sci 2019;49(3):894-8.
[Crossref] [Google Scholar] [PubMed]
- Eisenhauer EA, Verweij J. 11 New response evaluation criteria in solid tumors: RECIST guideline version 1.1. Eur J Cancer 2009;45(2):228-47.
[Crossref] [Google Scholar] [PubMed]
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136(5):E359-86.
[Crossref] [Google Scholar] [PubMed]
- Cremolini C, Antoniotti C, Rossini D, Lonardi S, Loupakis F, Pietrantonio F, et al. Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression vs. mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol 2020;21(4):497-507.
- Bao SM, Hu QH, Yang WT, Wang Y, Tong YP, Bao WD. Targeting epidermal growth factor receptor in non-small-cell-lung cancer: Current state and future perspective. Anti-Cancer Agents Med Chem 2019;19(8):984-91.
[Crossref] [Google Scholar] [PubMed]
- London M, Gallo E. Epidermal growth factor receptor (EGFR) involvement in epithelial-derived cancers and its current antibody-based immunotherapies. Cell Biol Int 2020;44(6):1267-82.
[Crossref] [Google Scholar] [PubMed]
- Yamanouchi K, Kuba S, Eguchi S. Hormone receptor, human epidermal growth factor receptor-2, and Ki-67 status in primary breast cancer and corresponding recurrences or synchronous axillary lymph node metastases. Surg Today 2020;50(7):657-63.
[Crossref] [Google Scholar] [PubMed]
- Dias MF, Blumenstein R, Russo J. Use of laser capture microdissection allows detection of loss of heterozygosity in chromosome 9p in breast cancer. Oncol Lett 2017;13(5):3831-6.
[Crossref] [Google Scholar] [PubMed]
- Martinelli E, Ciardiello D, Martini G, Troiani T, Cardone C, Vitiello PP, et al. Implementing anti-epidermal growth factor receptor (EGFR) therapy in metastatic colorectal cancer: Challenges and future perspectives. Ann Oncol 2020;31(1):30-40.
[Crossref] [Google Scholar] [PubMed]
- Kumar M, Joshi G, Chatterjee J, Kumar R. Epidermal growth factor receptor and its trafficking regulation by acetylation: Implication in resistance and exploring the newer therapeutic avenues in cancer. Curr Top Med Chem 2020;20(12):1105-23.
[Crossref] [Google Scholar] [PubMed]
- Kaufman NE, Dhingra S, Jois SD, Vicente MD. Molecular targeting of epidermal growth factor receptor (EGFR) and vascular endothelial growth factor receptor (VEGFR). Molecules 2021;26(4):1076.
[Crossref] [Google Scholar] [PubMed]
- Eberst L, Bailleux C, Bachelot T. Prevention of brain metastases in human epidermal growth factor receptor 2-positive breast cancer. Curr Opin Oncol 2020;32(6):555-60.
[Crossref] [Google Scholar] [PubMed]
- Masuda N, Kosaka N, Iwata H, Toi M. Palbociclib as an early-line treatment for Japanese patients with hormone receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer: A review of clinical trial and real-world data. Int J Clin Oncol 2021;26(12):2179-93.
[Crossref] [Google Scholar] [PubMed]
- Jain P, Arora T, Wadhwa N, Joshi MK. Human epidermal growth factor receptor 2 is frequently over-expressed in gastric carcinoma in north Indian patients. J Cancer Res Ther 2021;17(4):1136-7.
[Crossref] [Google Scholar] [PubMed]
 .
.



