- *Corresponding Author:
- Xiaowei Zhang
Department of Gynecology, The First Hospital of Hebei Medical University,050000, China,
E-mail: z381764542@163.com
| This article was originally published in a special issue, “Biomedical Research in Clinical and Preclinical Pharmaceutics” |
| Indian J Pharm Sci 2021:83(4)Spl issue “221-227” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the effect of electrical stimulation combined with pelvic floor muscle training on the improvement of urethral function in rats with stress urinary incontinence and to further study its possible mechanism. 48 rats were randomly divided into blank control group, model group, pelvic floor muscle training treatment group and electrical stimulation combined pelvic floor muscle training treatment group. The rehabilitation treatment group was treated with pelvic floor muscle training and combined electrical stimulation treatment after successful modeling. The sneeze test, urodynamics and pelvic floor muscle strength measurement were used to detect the rat urethra and pelvic floor muscle function. The expressions of vascular endothelial growth factor and extracellular-signal-regulated kinase 1/2 genes in urethral tissue cells were detected by reverse transcription-polymerase chain reaction. The rat sneeze test, urodynamics, pelvic floor muscle strength measurement, microvessel count and urethral histopathology results showed that the rat stress urinary incontinence model was successfully constructed and electrical stimulation combined with pelvic floor muscle training could significantly reduce the incidence of rate urinary incontinence and improve the pelvic floor muscle strength, the number of urethral capillaries and the repair of damaged vaginal nerves. Reverse transcription–polymerase chain reaction analysis showed that after rehabilitation treatment, the expressions of vascular endothelial growth factor and extracellularsignal- regulated kinase 1/2 were up-regulated and the up-regulation was more obvious in the combined electrical stimulation group. Electrical stimulation combined with pelvic floor muscle training could effectively improve the urethral function of rats and the action mechanism may be related to its activation of vascular endothelial growth factor and extracellular regulatory protein kinase extracellular-signalregulated kinase 1/2.
Keywords
Electrical stimulation, stress urinary incontinence, urethral function, vascular endothelial growth factor, extracellular-signal-regulated kinase 1/2
Stress urinary incontinence (SUI) refers to the involuntary leakage of urine caused by the increase of abdominal pressure, which often occurs in puerpera and elderly women and seriously affects the living quality of patients [1-3]. Studies have confirmed that moderate pudendal nerve electrical stimulation can promote cell activity, which is beneficial to urethral and vaginal tissue repair of SUI patients [4,5]. Electrical stimulation combined with pelvic floor training can effectively relieve the symptoms of SUI [6,7], which is an efficient clinical treatment scheme for SUI. Nevertheless, its mechanism is still unclear, which further hinders the development of treatment research. In view of this, this study explored the effect of electrical stimulation combined with pelvic floor training on urethral function of SUI rats and further studied its possible mechanism.
Materials and Methods
Experimental animals:
48 healthy specific pathogen-free (SPF) female rats, aged 1.5 to 2 mo, not pregnant or giving birth, weighing 180 to 220 g, were purchased from Beijing Weitong Lihua Co., Ltd. They were fed in the dark and light for 12 h alternately at room temperature and took food and water freely.
Methods:
Establishment of rat stress urinary incontinence model: After weighing 48 rats, 36 rats were randomly selected and anesthetized with 4 % chloral hydrate in abdominal cavity. The rat stress urinary incontinence model was established by vaginal dilation and pudendal nerve compression (VD+PNC). Vaginal dilatation: the head of 8F Foley catheter was placed 2 to 3 cm in rat vagina and 5 ml saline was extracted by syringe and injected into balloon. 150 g weight was suspended at the tail end and the rats were placed on a horizontal table top (ensuring that the pubic symphysis was tangent to the table edge, so that the vaginal tissue was uniformly stressed). After maintaining this state for 4 h, the water sac was slowly released and a small amount of sterile saline was injected along the vaginal opening. Then, the urinary catheter was removed; pudendal nerve compression: the rats were in prone position. A 1 cm transverse incision was made at the middle and lower 1/3 of the connecting line between the posterior superior iliac spine and ischial tubercle. And the muscle layers were passively separated. After the bilateral pudendal nerves were separated, the pudendal nerves (both sides) were clamped twice with micro forceps, with an interval of 5 s each time. The clamping lasted for 30 s each time. Muscles and skin were sutured sequentially. After that, the rates were fed naturally for later detection.
Experimental grouping: The rats were randomly divided into three groups, namely model group (without treatment), pelvic floor muscle training treatment group (pelvic floor muscle training group) and electrical stimulation combined with pelvic floor muscle training treatment group (combined electrical stimulation group), with 12 rats in each group. A blank control group was set up and the 12 rats were not modeled and treated. Pelvic floor muscle training: the vagina and anus of rats were stimulated by physical methods to make them contract naturally, thus achieving the purpose of pelvic floor muscle training. The training was carried once every day and each training lasted for 10 min. Combined electrical stimulation group: the electrodes were inserted into the posterior vaginal wall of female rats for electrical stimulation treatment (pulse wave electrical stimulation, current 0.3 mA, interval 0.1 ms, frequency 20 Hz). The treatment was carried out twice every week and each treatment lasted for 25 min. The treatment was given for 3 w.
Sneeze test: The rats were in supine position and the epidural catheter was placed into the bladder 2 to 3 cm after the urethral orifice was sterilized. After emptying the bladder, the epidural catheter was connected with the micropump to inject sterile physiological saline (12 ml/h) stained with methylene blue and the maximum bladder capacity was recorded when blue liquid overflowed from the urethral orifice. After emptying the rat bladder again, 1/2 of the maximum bladder volume of methylene blue stained normal saline was injected. A section of rat's beard was cut out and put into rat's nostril to stimulate it to cause sneezing reflex. After that, the rats were observed for blue liquid flowing out of the urethral orifice of rats. If there was liquid flowing out, it would be recorded as positive in sneeze test. If there is no liquid flowing out, it would be recorded as negative. The number of sneeze positive rats in each group was counted and the positive ratio was calculated.
Urodynamic test: One day before the test, the rats in each group were given bladder catheterization and the urodynamics was detected by urodynamics analyzer. The rats were in supine position. After emptying the bladder, they were injected with methylene blue stained normal saline (12 ml/h) by micropump. When the blue liquid overflowed from the urethral orifice and the pressure in the bladder increased rapidly, the bladder pressure at this time, namely leak point pressure (LPP), was recorded; the amount of saline injected was calculated as the maximum bladder capacity (MBC). Each rat was measured 3 times and the mean value was taken.
Pelvic floor muscle strength measurement: The rats were in supine position. The pelvic floor tissues were separated layer by layer. The body of pubococcygeus was exposed and was stimulated with platinum wire electrode (frequency once per second, voltage 5V, time 10 ms). The maximum muscle tension during single contraction was recorded. After testing, the pubococcygeus muscle was cut off and weighed and the ratio of single contraction force to muscle weight was calculated.
Microvessel density count of proximal urethra: After the rats were killed, the proximal urethra of the injection site was taken and continuous sections with thickness of 4 μm were made by hematoxylin-eosin (HE) staining. Microvessel counting (stained single endothelial cell cluster as a microvessel), namely microvessel density, was carried out in 5 visual fields under 200 times optical microscope. At least 3 samples were observed in each group and averaged.
Pathomorphological observation of pudendal nerve tissue in rats: Rats were anesthetized with 2 % pentobarbital abdominal cavity and fixed on the operating table. In prone position, transverse incision was taken at the middle and lower 1/3 level of the connecting line between the posterior superior iliac spine and ischial tubercle. The muscle layers were passively separated. The pudendal nerve tissue of rats was taken. After fixed with paraformaldehyde solution for 24 h, the tissue was dehydrated, embedded in paraffin, sliced and baked at 65°. After dewaxing, hydration, soaking in distilled water and HE staining, the tissue was cleared with xylene solution, sealed with neutral gum and dried. The sealing film was placed under a microscope to observe the histopathological changes of pudendal nerve in rats.
Detection of changes of expressions of vascular endothelial growth factor (VEGF) and extracellularsignal- regulated kinase (ERK) 1/2 by RT-PCR: Total RNA of anterior vaginal wall was extracted by conventional method. With mRNA as template, complementary DNA (cDNA) was amplified by reverse transcription reaction system and stored at -20°. The expressions of VEGF and ERK 1/2 were detected by reverse transcription–polymerase chain reaction (RT-PCR). PCR amplification conditions are: predenaturation at 94° for 2.5 min, denaturation at 95° for 25 s, annealing at 60° for 30 s, extension at 71° for 30 s, repeating for 40 times and extension at 72° for 60 s. 10 μl of amplification product and 6 μl of DGL-200 Maker were sampled at the same time and separated by 2.5 % agarose gel electrophoresis. The amplification results were analyzed and photographed by ultraviolet gel imaging system.
Statistical analysis:
All the data in this study were processed by statistical package for the social sciences (SPSS) 20.0 statistical analysis software. The measurement data were expressed by "mean±standard deviation"; variance analysis was used for comparison among multiple groups and least significant difference (LSD)-t test was used for comparison between groups; the counting data were expressed in percentage (%); p<0.05 indicated statistically significant difference.
Results and Discussion
None of the experimental animals died. Both the model group and the treatment group successfully completed the modeling operation. After the operation, the food intake, urine and feces of rats were normal and there was no infection at the modeling incision, which could meet the requirements of follow-up testing. There was no significant difference in hair of rats in each group.
The results of sneeze test in blank control group, model group, pelvic floor muscle training group and combined electrical stimulation group are shown in Table 1. It can be seen from Table 1 that compared with the blank control group, the positive rate of sneeze test in the model group was significantly increased and the difference was statistically significant (p<0.05). After rehabilitation, the positive rate of sneeze test in the treatment group (pelvic floor muscle training group and combined electrical stimulation group) was significantly lower than that in the model group, especially in the combined electrical stimulation group (p<0.05). The results showed that the model of stress urinary incontinence in rats was successfully established. Either pelvic floor muscle training or electrical stimulation could reduce the incidence of urinary incontinence and electrical stimulation combined with pelvic floor muscle training could improve the urethral function of rats better (Table 1).
| Group | Case | Positive | Negative | Positive rate |
|---|---|---|---|---|
| Control group | 12 | 0 | 12 | 0.00 % |
| Model group | 12 | 11 | 1 | 92.31 % |
| Pelvic floor muscle training group | 12 | 7 | 5 | 58.33 % |
| Combined electrical stimulation group | 12 | 3 | 9 | 25.00 % |
| F value | - | 13.64 | 13.51 | 16.27 |
| p value | - | <0.05 | <0.05 | <0.05 |
Table 1: Comparison of Sneeze Test Results of Rats in Each Group (n=48)
The urodynamic test results of blank control group, model group, pelvic floor muscle training group and combined electrical stimulation group are shown in Table 2. It can be seen from Table 2 that compared with blank control group, the LPP and the MBC of model group were significantly reduced and the difference was statistically significant (p<0.05). After rehabilitation, the LPP and MBC of treatment groups (pelvic floor muscle training group, combined electrical stimulation group) were higher than those of model group. The effect in combined electrical stimulation group was more obvious, with significant difference (p<0.05). The results further indicated that the rat model of stress urinary incontinence was successfully established. Either pelvic floor muscle training or electrical stimulation could improve the urethral function better and electrical stimulation combined with pelvic floor muscle training could improve the urethral function better (Table 2).
| Group | Case | Leakage point pressure LPP (cm H2O) | Maximum bladder capacity MBC (mL) |
|---|---|---|---|
| Control group | 12 | 38.86±4.13 | 2.51±0.16 |
| Model group | 12 | 25.21±3.71 | 1.24±0.13 |
| Pelvic floor muscle training group | 12 | 29.87±2.95 | 1.83±0.21 |
| Combined electrical stimulation group | 12 | 32.15±2.94 | 2.28±0.15 |
| F value | - | 3.64 | 3.51 |
| p value | - | <0.05 | <0.05 |
Table 2: Comparison of Urodynamic Test Results of Rats in Each Group (n=48)
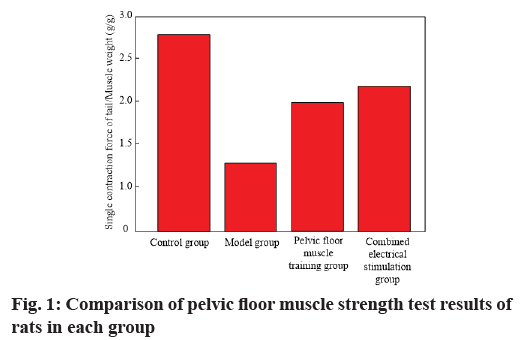
The results of pelvic floor muscle strength test in each group are shown in Table 3 and fig. 1. It can be seen from Table 3 that compared with blank control group, the ratio of single contraction force/muscle weight of pubococcygeus of model group was significantly reduced, with statistical significance (p<0.05). After rehabilitation treatment, the ratio of single contraction force/muscle weight of pubococcygeus in treatment groups (pelvic floor muscle training group, combined electrical stimulation group) was significantly higher than that in model group. The improvement effect in combined electrical stimulation group was more obvious, with significant difference (p<0.05). The results showed that rehabilitation therapy could effectively improve pelvic floor muscle strength of rats and electrical stimulation combined with pelvic floor muscle training had a better effect on improving pelvic floor muscle strength of rats (Table 3).
| Group | Case | Single contraction force/muscle weight of pubococcygeus muscle (g/g) |
|---|---|---|
| Control group | 12 | 2.72±0.13 |
| Model group | 12 | 1.33±0.07 |
| Pelvic floor muscle training group | 12 | 2.07±0.10 |
| Combined electrical stimulation group | 12 | 2.35±0.12 |
| F value | - | 4.61 |
| p value | - | <0.05 |
Table 3: Comparison of Pelvic Floor Muscle Strength Test Results of Rats in Each Group (n=48)
The effects of electrical stimulation combined with pelvic floor muscle training on the formation of microvessels in proximal urethra of rats are shown in Table 4. According to Table 4, compared with blank control group, the microvessel quantity of proximal urethra of model group rats decreased significantly and the difference was statistically significant (p<0.05). After rehabilitation treatment, the microvessel quantity of proximal urethra in treatment groups (pelvic floor muscle training group, combined electrical stimulation group) was significantly higher than that in the model group and the improvement effect in combined electrical stimulation group was more obvious, with significant difference (p<0.05). The results showed that rehabilitation therapy could promote the formation of microvessels in proximal urethra of rats and electrical stimulation combined with pelvic floor muscle training could improve the capability of microvessel formation better (Table 4).
| Group | Case | Microvessel quantity (hpf) |
|---|---|---|
| Control group | 12 | 18.65±1.21 |
| Model group | 12 | 10.43±2.37 |
| Pelvic floor muscle training group | 12 | 14.27±2.10 |
| Combined electrical stimulation group | 12 | 16.39±2.14 |
| F value | - | 3.64 |
| p value | - | <0.05 |
Table 4: Comparison of Microvessel Density in Proximal Urethra of Rats in Each Group (n=48)
HE staining results of pudendal nerves of rats in each group showed that the rats in blank control group had a large amount of pudendal nerves arranged neatly; after VD+PNC modeling, the pudendal nerves of model group rats were disordered and obvious nerve defects, twists and pulls could be observed; after rehabilitation treatment, the pudendal nerve of rats in treatment group could be partially damaged and repaired and nerve fibers gathered for healing at the defect. In addition, the electrical stimulation combined with pelvic floor muscle rehabilitation training had better healing effect. See Table 5 for the design of VEGF, ERK 1/2 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers.
| Premier name | Forward premier | Backward premier | Length (bp) |
|---|---|---|---|
| VEGF | 5′-GGAAGAGCTTCGACCAGCTG-3′ | 5′-GAGCCATTGTTAGTTTACAG-3′ | 132 |
| ERK 1 | 5′-AACCGAGCTTAGGCCAGCTG-3′ | 5′-AAGCCTTGACGCGTTTACAG-3′ | 128 |
| ERK 2 | 5′-AACCGAGCATTAGGCCAGCTG-3′ | 5′-AAGCCTTGACGGCTATACAG-3′ | 124 |
| GAPDH | 5′-CAGCATTGGAAGTGCTATGG-3′ | 5′-TAGAGCGAGTATGCATGACA-3′ | 138 |
Table 5: Primer Sequence Design in PCR Process
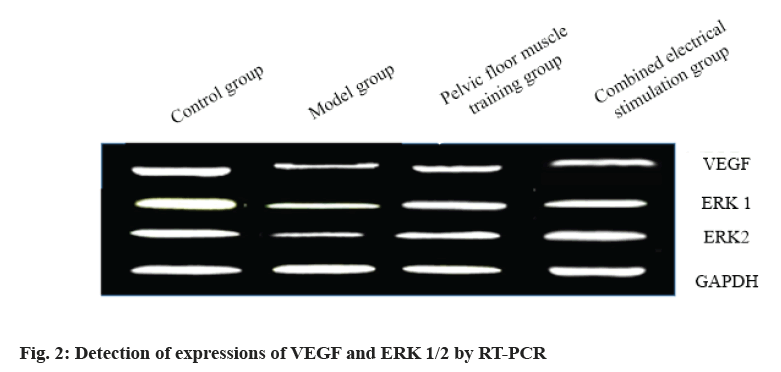
The expressions of VEGF and ERK 1/2 in the anterior urethra of rats are shown in Table 6 and fig. 2. Compared with blank control group, the expressions of VEGF and ERK 1/2 in model group decreased significantly and the difference was statistically significant (p<0.05). After rehabilitation treatment, the expressions of VEGF and ERK 1/2 in model group were significantly up-regulated compared with those in model group and the difference was statistically significant (p<0.05). At the same time, it is worth noting that the expressions of VEGF and ERK 1/2 in combined electrical stimulation group were significantly up-regulated. See Table 6.
| Index | VEGF (gray value) | ERK 1 (gray value) | ERK 2 (gray value) |
|---|---|---|---|
| Control group | 3.24±0.40 | 3.74±0.32 | 2.91±0.22 |
| Model group | 1.06±0.06 | 1.05±0.12 | 1.35±0.17 |
| Pelvic floor muscle training group | 2.36±0.21 | 2.51±0.46 | 2.01±0.16 |
| Combined electrical stimulation group | 2.89±0.32 | 2.93±0.42 | 2.43±0.21 |
| F value | 4.17 | 4.73 | 3.28 |
| p value | <0.05 | <0.05 | <0.05 |
Table 6: Detection of Expressions of VEGF and ERK 1/2 by RT-PCR
Stress urinary incontinence is a common chronic disease that seriously affects the living quality of middle-aged and elderly women. Birth trauma and low estrogen level are the two major causes of the disease, but its pathogenesis is unknown so far [8- 10]. At present, the common treatment methods are pelvic floor muscle training, electrical stimulation therapy, biofeedback therapy and drug therapy [11-13]. Vaginal electrical stimulation can indirectly stimulate nerves through polysynaptic reflex, causing passive contraction of pelvic floor muscles and improving urethral function [14,15]. Electrical stimulation combined with pelvic floor muscle training has a good therapeutic effect, but its mechanism is still unclear.
In view of this, VD+PNC method was used to expand pudendal nerve and press pudendal nerve in rats and the model of rat stress urinary incontinence was established. To verify the success of SUI model and the improvement of urethral function of SUI rats by electrical stimulation combined with pelvic floor muscle training, we used sneezing test, urodynamics and pelvic floor muscle strength measurement. Compared with blank control group, the positive rate of sneeze test in model group was significantly increased, while the leakage point pressure LPP, the maximum bladder capacity MBC and the ratio of single contraction force/muscle weight of pubococcygeus were significantly decreased, and the difference was statistically significant (p<0.05), suggesting that the model of rat SUI was successfully established. Further analysis showed that after rehabilitation treatment (pelvic floor muscle training combined with electrical stimulation), the positive rate of sneezetest could be reduced to a certain extent and the ratio of single contraction force/muscle weight of LPP, MBC and pubococcygeus muscle could be increased. And the improvement effect of combined electrical stimulation was the best, with significant difference (p<0.05); it suggests that either pelvic floor muscle training or electrical stimulation can reduce the incidence of urinary incontinence, improve urethral function and improve pelvic floor muscle strength and electrical stimulation combined with pelvic floor muscle training can improve urethral function of rats better. A large number of studies have shown that the number of urethral microvessels and pudendal nerve tissue morphology in rats can reflect the urethral tissue condition to a certain extent [16,17]. After that, we have further examined the formation of proximal urethral microvessels and vaginal nerve tissue morphology in rats. The results of microvessel density counting and HE staining showed that rehabilitation treatment could promote the formation of proximal urethral microvessels and the healing of pudendal defect nerves in rats and the treatment effect of combined electrical stimulation was better. It is suggested that electrical stimulation combined with pelvic floor muscle training can effectively promote the regeneration of urethral microvessels and repair of damaged vaginal nerves in rats. Studies have proved that VEGF, as a highly specific vascular endothelial growth factor, can promote the migration, proliferation and angiogenesis of vascular endothelial cells [18,19]; ERK1/2, an extr tinence in rats. To further explore the mechanism of electrical stimulation combined with pelvic floor muscle training in the recovery of SUI in rats, we further detected the expressions of VEGF acellular regulatory protein kinase, is the main signal pathway of cell stress and injury response [20], which can regulate basic cell processes. Both of them play an important role in the occurrence, development and recovery of stress urinary incon and ERK 1/2 in the anterior urethral tissues of rats by RT-PCR. The results showed that after rehabilitation treatment, the expressions of VEGF and ERK 1/2 were significantly up-regulated compared with model group and the up-regulation was especially significant in combined electrical stimulation group. The difference was statistically significant (p<0.05).
To sum up, this study showed that electrical stimulation combined with pelvic floor muscle training could significantly improve the urethral function of SUI rats and its mechanism may be related to its activation of VEGF and ERK1/2 signaling pathway. However, its specific mechanism is still unclear and further in-depth study is needed. The current research results showed that electrical stimulation significantly up-regulated the expressions of VEGF and ERK 1/2, providing some experimental data and theoretical support for the application of electrical stimulation therapy in SUI clinical treatment.
Conflicts of interest:
The authors declared no conflicts of interest.
References
- Cheng H, Gu RR, Wu LP. Current situation and influencing factors of knowledge-attitude-practice of pelvic floor muscle training in women with postpartum stress urinary incontinence, Chin J Mod Nurs 2021;27(9):1185-9.
- Wang LP, Wang F, Xu AZ. Analysis of the pelvic floor muscle function and its effect factors among 1270 postpartum women. Maternal Child Health Care China 2014;29(1):29-31.
- Feng SN, Lian TY. Investigation on the current situation of pelvic floor muscle function among postpartum women. Maternal Child Health Care China 2015;30(31):5449-51.
- Wu HQ, Qian QY, Qian QY, Zhou BB, Yu XX, Cao JJ. Survey and analyze of 1 400 women's pelvic floor muscle strength, J Pract Med Technique 2013;20(10):1045-7.
- Su TS, Liu BY, Liu ZS, Chen YL, Zhang W, Chu HR, et al. Electroacupuncture versus Pelvic floor muscle training for treatment of female stress urinary incontinence: A multipul-centered, randomized controlled trial. J Tradit Chin Med 2021;62(5):414-8.
- Sun Z, Wang X, Lang J, Xu T, Zhang Y, Kang J, et al. Comparison of outcomes between single?incision sling and transobturator sling for treating stress urinary incontinence: A 10?year prospective study. Neurourol Urodyn 2019;38(7):1852-8.
- Lo TS, Chua S, Kao CC, Uy-Patrimonio MC, Ibrahim R, Tan YL. Five-year outcome of MiniArc single-incision sling used in the treatment of primary urodynamic stress incontinence. J Minim Invasive Gynecol 2018;25(1):116-23.
- Haylen BT, de Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, et al. An international urogynecological association (IUGA)/international continence society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J 2010;21(1):5-26.
- Wang XF, Wu SH, An J. Clinical study on pelvic floor muscles exercises combining with acupuncture to treat female patients with stress urinary incontinence. Chin Nurs Res 2011;25(18):1656-7.
- Schäfer W, Abrams P, Liao L, Mattiasson A, Pesce F, Spangberg A, et al. Good urodynamic practices: uroflowmetry, filling cystometry and pressure?flow studies. Neurourol Urodyn 2002;21(3):261-74.
- Palmieri S, Frigerio M, Spelzini F, Manodoro S, Milani R. Risk factors for stress urinary incontinence recurrence after single?incision sling. Neurourol Urodyn 2018;37(5):1711-6.
- Spelzini F, Frigerio M, Regini C, Palmieri S, Manodoro S, Milani R. Learning curve for the single?incision suburethral sling procedure for female stress urinary incontinence. Int J Gynaecol Obstet 2017;139(3):363-7.
- Mostafa A, Lim CP, Hopper L, Madhuvrata P, Abdel-Fattah M. Single-incision mini-slings versus standard midurethral slings in surgical management of female stress urinary incontinence: an updated systematic review and meta-analysis of effectiveness and complications. Eur Urol 2014;65(2):402-27.
- Ding ZY, Ding QQ, Wang AH, Zhao Y, Zuo SQ, Zhang RL, et al. Application of teach-back method combined with the theory of goal attainment in pelvic floor muscle training of elderly female patients with stress urinary incontinence. Henan Med Res 2016;30(3):410-4.
- Zalewski M, Ko?ody?ska G, Zalewska A, Andrzejewski W. Comparative Assessment of Female Sexual Function Following Transobturator Midurethral Sling for Stress Urinary Incontinence. Int J Environ Res Public Health 2021;18(5):2286.
- Fuentes-Aparicio L, Balasch-Bernat M, López-Bueno L. Add-On Effect of Postural Instructions to Abdominopelvic Exercise on Urinary Symptoms and Quality of Life in Climacteric Women with Stress Urinary Incontinence. A Pilot Randomized Controlled Trial. Int J Environ Res Public Health 2021;18(3):928.
- Chen Y, Cai Q, Pan J, Zhang D, Wang J, Guan R, et al. Role and mechanism of micro-energy treatment in regenerative medicine. Transl Androl Urol 2020;9(2):690-701.
- Richter A, Alexdottir MS, Magnus SH, Richter TR, Morikawa M, Zwijsen A, et al. EGFL7 mediates BMP9-induced sprouting angiogenesis of endothelial cells derived from human embryonic stem cells. Stem Cell Rep 2019;12(6):1250-9.
- Liu ZH, Wei LH, Ling FL, Luo QP. Effects of King Interaction standard theory on the compliance with pelvic floor muscle exercises in elderly women with stress urinary incontinence. Med High Vocat Edu Mod Nurs 2020;3(2):119-22.
- Wei LS. Application of system health education combined with anti-resistance training of pelvic floor muscle in patients with stress urinary incontinence. Nurs Pract Res 2019;16(16):90-2.