- *Corresponding Author:
- Shaohong Li
Department of Critical Care Medicine,
Dongguan Tung Wah Hospital,
No. 1, Dongcheng East Road,
Dongguan Guangdong 523110,
China
E-mail: szhx12@163.com
| This article was originally published in a special issue, “Diagnostic and Therapeutic Advances in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2021:83(5) spl issue “45-54” |
Abstract
To investigate the role of soluble programmed death protein 1 in immunosuppression of sepsis and its prognostic value. The patients were admitted from our hospital and divided into sepsis group (110), septic shock group (81) and control group (52). The relationship of soluble programmed death protein 1 and prognosis in patients with sepsis and septic shock were detected. On the 1st d and 7th d, the lymphocyte count, percentage and total T lymphocyte ratio were significantly lower and soluble programmed death protein 1 level was significantly higher than those of in the control group (p<0.05). The level of soluble programmed death protein 1 and the lymphocyte count and percentage on the 7th d were significantly higher than that of the 1st d (p<0.05). Compared with the 1st d, the immunoglobulin G, immunoglobulin A, immunoglobulin M and complement C3 were significantly increased (p<0.05). Soluble programmed death protein 1 was independently correlated with sequential organ failure score (R2=0.08, F=6.39, p<0.05) and the acute physiology and chronic health evaluation alpha score (OR=1.17, p=0.01), programmed death protein 1 level (OR=1.46, p=0.01) on the 1st d are independent risk factors for death in patients. The expression level of soluble programmed death protein 1 is related to the severity of the disease. Soluble programmed death protein 1 can effectively predict the 28 d prognosis of patients with sepsis and septic shock.
Keywords
Sepsis, immunosuppression, T lymphocytes, soluble programmed death protein 1
Sepsis is a life-threatening disease with multiple organ dysfunction caused by dysregulation of host response to pathogen infection [1-3], which is often accompanied by immune dysfunction and systemic inflammatory response syndrome. They are usually in critical condition with rapid progression and high fatality rate [4,5]. The high fatality rate and high medical cost of sepsis have brought serious health risk and economic pressure to the society and family, which has attracted extensive attention of researchers from all circles of society. In order to more accurately evaluate the severity of the disease progression of patients with sepsis, a deeper study on the pathogenesis of sepsis patients has become an urgent need of the academic community.
The pathophysiological mechanism of sepsis is complex, among which the main pathological mechanism is the immunosuppressive reaction caused by the imbalance between pro-inflammatory and anti-inflammatory responses in the course of disease progression [6]. As the most powerful antigen-presenting cell, dendritic cells (DC) will have a great impact on the immune status of the body after being suppressed, which is of great significance for the study of the mechanism of immunosuppression [5]. Studies have shown that in patients with sub-acute sepsis, the body enters the stage of immunosuppression and T lymphocyte (T cell) function is inhibited [7,8]. At this stage, the number of DCs is significantly reduced, a large number of tolerant DCs are generated and the activation of DC functions met with obvious obstacles [9].
Studies have shown that the overexpression of programmed death protein 1 (PD-1) is an important reason for the functional inhibition of DC and anti- PD-1 has achieved significant effects in the treatment of tumors. Similar to tumor, sepsis is also a disease of abnormal immune response. Anti-PD-1/programmed death-ligand 1 (PD-L1) has shown obvious efficacy in relevant animal experiments, but the relevant antibodies for the treatment of sepsis patients are currently in clinical trials and the efficacy is still unclear [10]. Previous study demonstrate that plasma concentrations of soluble programmed death protein 1 (sPD-1) was correlated to severity of disease and a poor prognosis. However the relationships between sPD-1 and subtypes of various immune cells as well as immunoglobulins (Igs) in sepsis were not investigated. In this study, the dynamic changes of serum sPD-1 in patients with sepsis and septic shock were observed and the relationship between sPD-1 and lymphocyte count, lymphocyte subpopulation analysis and immunoglobulin level was observed, aiming to reveal the relationship between sPD-1 and humoral immunity and cellular immunity in patients with sepsis. At the same time, the relationship between sPD-1, disease severity and 28 d mortality of patients with sepsis was observed to preliminarily explore the value of sPD-1 in evaluating the prognosis of sepsis.
Materials and Methods
General clinical data:
The prospective observational method was adopted in this study. Subjects were recruited from the intensive care unit (ICU) and electronic intensive care unit (eICU) patients of our hospital from June 2018 to January 2021. 52 healthy volunteers were recruited as a control group. A total of 209 cases were collected in the sepsis and septic shock group, of which 10 cases were excluded because the measured values of related indicators exceeded the measured range, 3 cases were excluded because they were diagnosed as tumor based on their subsequent pathological results and 5 cases were excluded after because they accepted at least 1 w of treatment history outside the hospital or other departments before admission to the ICU. In the sepsis group, there were 110 patients, including 70 males and 40 females. Among them, 46 patients stayed in the ICU longer than 3 d and 64 patients stayed in the ICU longer than 7 d. The average ICU stay was 5.5±2.9 d. There were 81 patients in the septic shock group, including 55 males and 26 females. Among them, 15 patients stayed in the ICU longer than 3 d and 41 patients stayed in the ICU longer than 7 d. The average ICU stay was 7.3±4.1 d. In the general operation control group, there were 30 patients, including 21 males and 9 females, with an average age of 57.53±4.57 y. The survival of patients with sepsis and septic shock group after 28 d were followed up and the patients were divided into survival group (170 cases) and death group (39 cases).
Research method:
Record general clinical data of the object, such as age, gender, diagnosis, basic diseases (abdominal surgery, bone surgery, etc.,) the main source of infection (abdominal cavity infection, pulmonary infection, blood infection), etc., documented patients into ICU 24 h acute physiology and chronic health evaluation alpha score (APACHE ? score), sequential organ failure score (SOFA score), mechanical ventilation, ICU length of hospital stay, follow-up 28 d survival situation.
On the 1st d of the healthy control group, peripheral blood was collected for blood routine analysis, lymphocyte subsets analysis, IgG, IgM, IgA+complement components (C3, C4). In the sepsis group and septic shock group, the inflammatory indicators were measured and recorded on the 1st d of ICU: hypersensitive C-reactive protein (hypersensitive CRP), absolute value of white blood cells, percentage of neutrophils and procalcitonin (PCT). Immune indicators: lymphocyte count, lymphocyte percentage, lymphocyte subpopulation analysis, IgG, IgM, IgA+C3, C4; other indicators include lactic acid in arterial blood gas. Blood routine, hypersensitive CRP, PCT, lymphocyte subpopulation analysis, IgG, IgM, IgA+C3, C4 were measured in patients with sepsis and septic shock group on the 7th d in ICU. In the healthy control group, only the samples from d 1 were collected for sPD-1 measurement. Specimens of the sepsis group and the septic shock group were collected on the 1st and 7th d of admission to ICU to measure sPD-1.
Specimen collection and monitoring methods:
On the 1st , 3rd and 7th d in ICU, 3 ml of peripheral blood was collected using heparin anticoagulant tube and centrifuged in a centrifuge at 1500 r/min for 15 min to separate the plasma. The upper samples were collected using pipetting gun and placed at -30° refrigerator for temporary storage immediately and stored in a -80° refrigerator within a week. All the specimens were tested for sPD-1 in the same batch. Plasma sPD-1 content was determined by enzyme-linked immunosorbent assay (ELISA) and the experimental procedures were strictly in accordance with the kit instructions.
Determination and analysis of lymphocyte subsets:
3 to 5 ml of whole blood samples were taken, cluster of differentiation 3 (CD3)/CD4/CD8 antibodies were added into the flow tube, the blood samples were added into the flow tube and mixed, and incubated at room temperature and out of light for 30 min. Then added 2 ml of red blood cell lysate to dissolve the red blood cells, centrifuge, discard the supernatant and wash twice. Finally, added 0.5 ml of phosphate buffered saline (PBS) and test on the flow cytometer (test on the same day).
Statistical analysis:
Statistical package for the social sciences (SPSS) 21.0 software was used for data processing and analysis in this study. The measurement data of normal distribution were expressed as mean±standard deviation and two independent sample t test was used for comparison between groups. One way analysis of variance (ANOVA) was used to compare the mean values of multiple groups. Enumeration data were described by frequency and comparison between groups was performed by chi-square test. p<0.05 was considered statistically significant. Pearson or Spearman correlation analysis was performed in comparison between sPD- 1 and APACHE LI score, SOFA score and prognostic indicators. p<0.05 was considered statistically significant. Multivariate logistic regression analysis was used to determine the prognostic factors and p<0.05 was considered statistically significant. Then, the area under the receiver-operating characteristic (ROC) curve (AUC) of sPD-1 and prognosis in patients with sepsis and septic shock was evaluated.
Results and Discussion
There was no significant difference in age and gender composition among the healthy control group, sepsis group and septic shock group, and there was no statistical significance in the basic diseases between the latter two groups (p>0.05). The APACHE II score, SOFA score and blood lactic acid score in the septic shock group were higher than those in the septic shock group, but the differences were not statistically significant (p>0.05). There were no significant differences in the source of infection, length of stay in ICU and mechanical ventilation between the sepsis group and the septic shock group (p>0.05), as shown in Table 1.
| Subject | Sepsis (n=110) | Septic shock (n=81) | Healthy control (n=52) | χ2/F | p |
|---|---|---|---|---|---|
| Age (y) | 59.6±18.4 | 59.7±17.9 | 59.1±16.7 | 0.14 | >0.05 |
| Male (%) | 70 (63.6) | 55 (67.9) | 19 (63.3) | 0.75 | >0.05 |
| Disease classification | 0.94 | >0.05 | |||
| Internal Medicine | 86 (78.2) | 55 (67.9) | - | ||
| Surgery | 24 (21.8) | 26 (32.1) | - | ||
| Basic disease | 0.81 | >0.05 | |||
| Hypertension | 29 (25.9) | 20 (24.1) | - | ||
| Diabetes | 31 (27.7) | 24 (28.9) | - | ||
| COPD | 13 (11.6) | 11 (13.3) | - | ||
| Heart disease | 16 (14.3) | 13 (15.7) | - | ||
| Other | 12 (10.7) | 7 (8.4) | - | ||
| Source of infection | 1.12 | >0.05 | |||
| respiratory tract | 34 (30.9) | 25 (30.9) | - | ||
| Urinary tract | 27 (24.5) | 22 (27.2) | - | ||
| Hepatobiliary system | 21 (19.1) | 18 (22.2) | - | ||
| Bloodstream infections | 13 (11.8) | 9 (11.1) | - | ||
| Skin tissue | 7 (6.4) | 5 (6.2) | - | ||
| Other | 8 (7.3) | 2 (2.5) | - | ||
| Total length of stay (d) | 19.7 (8.1) | 14.1 (6.2) | - | 11.32 | >0.05 |
| Length of stay in the ICU (d) | 5.5 (2.9) | 7.3 (4.1) | - | 18.73 | >0.05 |
| APACHE II score | 22.8 (1.5) | 17.3 (1.2) | - | 27.55 | >0.05 |
| SOFA score | 10 (2.0) | 8.0 (1.5) | - | 54.88 | >0.05 |
| LAC mmol/l | 1.2 (0.5) | 1.6 (0.4) | 1.64 | >0.05 |
Table 1: Comparison of General Clinical Data among the Three Groups
On the 1st d of admission to ICU, lymphocyte count, lymphocyte percentage and total T lymphocyte ratio in sepsis and septic shock group were significantly lower than those in healthy control group. The level of sPD- 1 was significantly higher than that of healthy control group (p<0.05). There were no significant differences in CD8+ T cell %, CD4+ T cell % and helper/suppressor T cell ratio among the three groups (p>0.05). In septic shock group, besides sPD-1 was higher than that in sepsis group (p<0.05), there was no difference in other indexes between the two groups (p>0.05). On the 7th d after admission to ICU, sPD-1 in septic shock group was still significantly higher than that in control group and sepsis group (p<0.05). There was no significant difference in other remaining indexes between the sepsis group and the septic shock group (p>0.05). The proportion of total T lymphocytes in the septic shock group was lower than that in the control group and the proportion of CD8+ T lymphocytes was significantly lower than that in the control group (p<0.05). There were no significant differences in lymphocyte count, percentage of lymphocytes, proportion of CD8+ T cells and ratio of helper/suppressor T cells among the three groups (p>0.05). There was no significant difference between the control group and the sepsis group (p>0.05) as shown in Table 2.
| Subject | Time | Healthy | Sepsis | Septic shock |
|---|---|---|---|---|
| PD 1 | 1st d in ICU | 7.54±4.13 | 8.68±4.33* | 9.86±7.15*# |
| 7th d in ICU | 7.61±4.27 | 10.08±4.89* | 10.51±7.12*# | |
| Lymphocyte Count×109/l | 1st d in ICU | 1.31±0.10 | 0.78±0.08* | 0.68±0.08* |
| 7th d in ICU | 1.24±0.22 | 1.05±0.20 | 1.13±0.21 | |
| Percentage of lymphocytes (%) | 1st d in ICU | 15.06±2.39 | 9.33±1.45* | 6.75±0.78* |
| 7th d in ICU | 15.06±2.39 | 12.01±1.28 | 10.21±1.01 | |
| Total T lymphocyte % | 1st d in ICU | 63.35±15.48 | 51.98±19.11 | 51.00±23.10 |
| 7th d in ICU | 63.35±15.48 | 54.10±19.37 | 43.90±15.27* | |
| CD8+ T % | 1st d in ICU | 26.79±2.28 | 25.90±1.82 | 23.10±2.64 |
| 7th d in ICU | 26.79±2.28 | 28.28±2.97 | 18.36±2.23 | |
| CD4+ T % | 1st d in ICU | 31.12±2.13 | 24.29±2.12 | 27.00±3.06 |
| 7th d in ICU | 31.12±2.13 | 27.625±1.32 | 24.85±4.03 | |
| Helper/suppressor T cell ratio | 1st d in ICU | 1.36±0.27 | 1.11±0.15 | 1.71±0.33 |
| 7th d in ICU | 1.36±0.27 | 1.53±0.21 | 1.38±0.22 |
Note: *p<0.05 compared with the healthy group; #p<0.05 compared with the sepsis group
Table 2: Comparison Of Spd-1, Lymphocyte Count and Lymphocyte Subsets between the Three Groups before and after Treatment
Compared with the control group, on the 1st d in ICU, immunoglobulin IgG and IgM were decreased in both the septic shock group and the sepsis group. Complement C3 was decreased only in septic shock group (p<0.05). There were no significant differences in immunoglobulin IgA, complement C4, Natural killer (NK) cell percentage and total B lymphocyte percentage among the four groups (p>0.05). Compared with the septic shock group, only C3 reduced (p<0.05) in sepsis group and no difference was found in other indicators. Compared with the control group, immunoglobulin IgG, IgM and IgA in the septic shock group were all increased on the 7th d after the patients were admitted to ICU, but only IgA was increased with statistical significance (p<0.05). There were no signific ant differences in the above indexes between the sepsis group and the control group (p<0.05). There were no significant differences in the proportion of complement C4, NK percentage and total B lymphocyte percentage among the three groups (p>0.05), as shown in Table 3.
| Subject | Time | Healthy | Sepsis | Septic shock |
|---|---|---|---|---|
| Ig G (g/l) | 1st d in ICU | 9.11±0.73 | 7.03±0.41* | 6.55±0.47* |
| 7th d in ICU | 9.11±0.73 | 9.34±1.26 | 11.90±1.15 | |
| Ig A (g/l) | 1st d in ICU | 1.77±0.16 | 1.88±0.19 | 1.64±0.16 |
| 7th d in ICU | 1.77±0.16 | 2.20±0.23 | 2.74±0.20* | |
| IgM (g/l) | 1st d in ICU | 0.94±0.17 | 0.61±0.03* | 0.53±0.05* |
| 7th d in ICU | 0.94±0.17 | 0.89±0.21 | 2.21±0.76* | |
| C3 (g/l) | 1st d in ICU | 0.84±0.06 | 0.75±0.04 | 0.57±0.21 |
| 7th d in ICU | 0.84±0.06 | 1.13±0.15 | 0.93±0.04 | |
| C4 (g/l) | 1st d in ICU | 0.21±0.04 | 0.21±0.03 | 0.18±0.01 |
| 7th d in ICU | 0.21±0.04 | 0.66±0.32 | 0.26±0.05 | |
| NK cell (%) | 1st d in ICU | 20.94±2.68 | 26.41±3.42 | 27.68±3.28 |
| 7th d in ICU | 20.94±2.68 | 23.40±7.81 | 31.14±4.22 | |
| Total B lymphocytes percentage (%) | 1st d in ICU | 15.04±2.13 | 17.77±2.40 | 16.70±2.02 |
| 7th d in ICU | 15.04±2.13 | 10.53±3.18 | 13.56±3.35 |
Note: *p<0.05 compared with the healthy group; #p<0.05 compared with the sepsis group
Table 3: Comparison of Immunoglobulin, Complement, Nk Cells Percentages and Total B Lymphocytes Percentages in Different Phases in Each Group
The level of sPD-1 in death group on 7th d was significantly higher than that on 1st d (p<0.05). There was no significant difference on the 1st and 7th d in dynamic monitoring of sPD-1 in the survival group (p>0.05). The lymphocyte count and lymphocyte percentage in survival group were significantly higher on 7th d than on 1st d (p<0.05). There was no significant difference in lymphocyte count between the 1st and 7th d in the death group (p>0.05). There was no significant difference in the dynamic monitoring of lymphocyte subsets between the death group and the survival group (p>0.05), as shown in Table 4.
| Subject | Time | Death group | Survival group |
|---|---|---|---|
| PD 1 | 1st d in ICU | 8.61±3.88 | 8.94±3.93 |
| 7th d in ICU | 16.22±4.34* | 9.91±4.23 | |
| Lymphocyte Count×109/l | 1st d in ICU | 0.74±0.12 | 0.72±0.07 |
| 7th d in ICU | 1.04±0.51 | 1.47±0.17* | |
| Percentage of lymphocytes (%) | 1st d in ICU | 6.08±2.17 | 6.99±1.19 |
| 7th d in ICU | 4.99±0.78 | 12.01±1.11* | |
| Total T lymphocyte % | 1st d in ICU | 53.24±13.16 | 52.76±18.14 |
| 7th d in ICU | 49.81±17.09 | 51.14±16.91 | |
| CD8+T cell % | 1st d in ICU | 22.98±4.83 | 26.97±4.54 |
| 7th d in ICU | 20.63±3.85 | 25.45±4.49 | |
| CD4+T cell % | 1st d in ICU | 22.78±6.23 | 22.46±2.46 |
| 7th d in ICU | 19.40±5.80 | 22.68±3.62 | |
| Helper/suppressor T cell ratio (%) | 1st d in ICU | 1.17±0.05 | 1.44±0.51 |
| 7th d in ICU | 1.08±0.08 | 1.19±0.22 |
Note: *p<0.05 compared with the 1st d
Table 4: Changes of Spd-1, Lymphocyte Count, Lymphocyte Percentage and Lymphocyte Subsets in the Survival and Death Groups
Compared with the 1st d in ICU, the levels of immunoglobulin IgG, IgA, IgM and complement C3 in the survival group on the 7th d in ICU were significantly increased (p<0.05), while there was no significant difference in complement C4. There were no significant differences in IgG, IgA, IgM, complement C3 and complement C4 in the death group on the 7th d of ICU (p>0.05) as shown in Table 5.
| Subject | Time | Death group | Survival group |
|---|---|---|---|
| Ig G (g/l) | 1st d in ICU | 7.73±0.70 | 5.61±0.63 |
| 7th d in ICU | 8.00±0.95 | 10.57±0.96* | |
| Ig A (g/l) | 1st d in ICU | 2.37±0.64 | 1.39±0.16 |
| 7th d in ICU | 2.48±0.64 | 2.56±0.16* | |
| IgM (g/l) | 1st d in ICU | 0.79±0.77 | 0.46±0.08 |
| 7th d in ICU | 0.77±0.26 | 1.24±0.21* | |
| C3 (g/l) | 1st d in ICU | 0.86±0.07 | 0.62±0.07 |
| 7th d in ICU | 1.08±0.12 | 0.96±0.06* | |
| C4 (g/l) | 1st d in ICU | 0.23±0.03 | 0.22±0.05 |
| 7th d in ICU | 0.27±0.02 | 0.23±0.02 |
Note: *p<0.05, compared with the 1st d
Table 5: Dynamic Monitoring of Immunoglobulin and Complement in Survival Group and Death Group
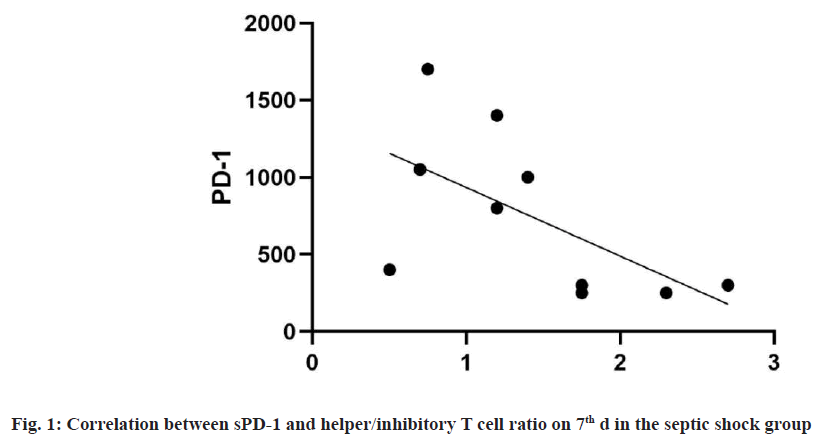
In the septic shock group, sPD-1 was negatively correlated with the ratio of helper/inhibitory T cells on 7th d (the correlation coefficient was -0.66 (p=0.038)). There was no significant correlation between sPD-1 and lymphocyte count, lymphocyte percentage, total T %, CD4+ % and CD8+ % as shown in fig. 1.
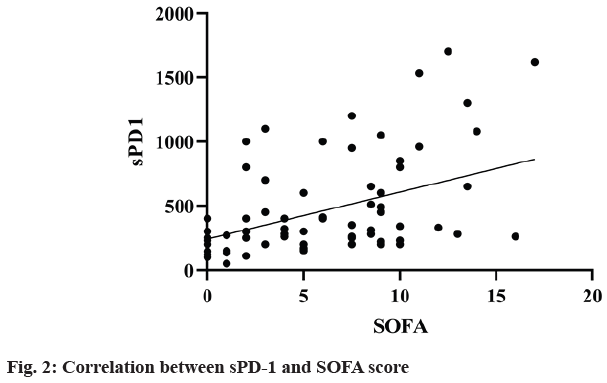
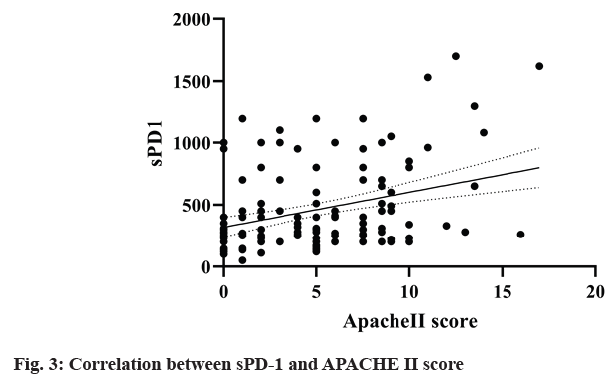
The correlation coefficient between sPD-1 and SOFA score and APACHE II score in survival group (n=170) and death group (n=39) was 0.027 (p=0.014). Linear regression analysis showed that sPD-1 was independently correlated with SOFA score (R2=0.18, F=36.47, p<0.0001) (fig. 2) and APACHE II score (R2=0.10, F=19.25, p<0.0001) (fig. 3). It suggested that sPD-1 was independently associated with survival and death.
The PD 1, the APACHE II score, SOFA score, allergic CRP, LAC and other variables were included in the logistic regression model, the results show that in the 1st d in ICU, APACHE II score (OR=1.17, p=0.01) and PD 1 expression level (OR=1.46, p=0.01) is the independent risk factors of death in patients with sepsis or sepsis shock, CRP was not found to be the independent risk factor of death in patients with sepsis or sepsis shock.
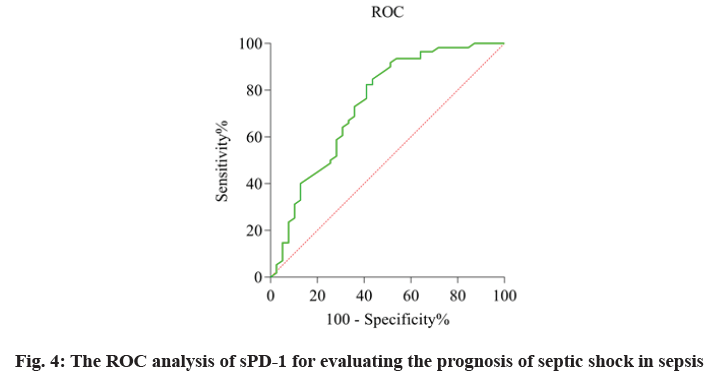
The relationship of sPD-1 level and prognosis in patients with sepsis and septic shock was defined as the AUC and was evaluated by using the ROC analysis (fig. 4). The AUC values of sPD-1 was 0.74. These data suggested sPD-1 can effectively predict the prognosis of patients with sepsis and septic shock as shown in Table 6.
| Variable | Wald χ2 value | p value | OR value | 95 % CI |
|---|---|---|---|---|
| APACHE II score | 10.90 | 0.01 | 1.17 | ( 1.07, 1.29) |
| PD1 | 12.37 | 0.01 | 1.46 | ( 1.19, 1.73) |
Table 6: Logistic Regression Analysis of the Prognosis of Patients with Sepsis and Septic Shock
Sepsis is characterized by high morbidity, high mortality and high cost. With the development of medical technology, the understanding and treatment of sepsis has become more and more profound. However, the current treatment programs mainly include anti-infection and organ function protection, etc. Although some progress has been made [11,12], the expected effect is still not achieved. The pathogenesis of sepsis is complex and immunosuppression is one of the pathophysiological mechanisms of sepsis [13]. Early the science society think sepsis was characterized by excessive inflammatory response in early stage [14], later developed into an immunosuppressive state. However, recent studies have found that in the early stage of sepsis, proinflammatory response is the main manifestation [15], but anti-inflammatory response has also begun to play a role. With the continuous consumption of inflammatory mediators and the continuous apoptosis of immune cells, patients enter the immunosuppressed state. Some modulatory therapies targeting immune function have made some progress in animal models, providing a new direction for the treatment of sepsis [16-18]. NK cells are an important component of innate immunity and play immunomodulatory roles through their natural killing [19]. B lymphocytes mediate humoral immunity. In this study, on the 1st d and 7th d B lymphocytes and NK cells proportion of sepsis and sepsis shock group have no obvious change compared with healthy controls and normal operation control group. There was no significant change in the proportion of B lymphocytes and NK cells in patients with toxic shock between entering the ICU on 1st d and 7th d, suggesting that there was no significant difference in the immunity mediated by NK cells and B lymphocytes in sepsis.
Immunoglobulin plays an important role in humoral immunity against infection [20]. The results of this study showed that patients with sepsis and septic shock showed a decrease in IgG and IgM on the 1st d of admission to ICU, while IgA did not change. With the extension of hospitalization time for septic shock, IgG and IgA gradually increased, and IgM also increased, but did not reach statistical significance. It suggested that the humoral immune dysfunction appeared in patients with sepsis in the early stage, but recovered with time lasting and was not related to the prognosis of the disease. In this study, sPD-1 was not correlated with the proportion of total B lymphocytes, suggesting that the decrease of immunoglobulin in the early stage of sepsis had nothing to do with lymphocyte apoptosis. Immunoglobulins play an important role in humoral immunity against infection, so hypoimmunoglobulinemia often appears in sepsis. Shankar-Hari et al. [20] found that the reduction of immunoglobulin in sepsis was the most common, exceeding 50 % and no correlation was found between its reduction and the risk of death. In this study, the immunoglobulin of the sepsis and septic shock groups on the 1st d was significantly decreased and the decrease of IgG was statistically significant (p<0.05), and no correlation with adverse risk was found. The decrease of IgG in sepsis was considered to be caused by excessive consumption of immunoglobulin, decreased secretion capacity or vascular endothelial injury to leakage, etc., [21]. In this study, it was found that the IgA value of patients in the septic shock group was significantly increased on the 7th d (p<0.05), which was consistent with the findings of Giamarellos-Bourboulis et al. [22]. Complement C3 is activated by the classical pathway or the bypass pathway and is consumed to play the role of anti-pathogen. In this study, there was a significant decrease in complement C3 in the septic shock group on the 1st d, indicating that the patients with septic shock consumed more complement. This result is consistent with Stover et al. [23]. There was no significant difference in complement C3 and C4 between the septic shock group and the normal operation control group at the 7th d (p>0.05). The results showed that the complement C3 could reflect the severity of the disease in the early stage, but with the development of the disease, with the control of infection and the recovery of the humoral immune system, the complement system also returned to normal. In conclusion, the immunoglobulin and complement significantly changed in the early stage of sepsis and septic shock, and were involved in the early stage of sepsis. However, as the disease progressed to the 7th d, complement C3 returned to normal, but immunoglobulin was still abnormal, so immunoglobulin may be involved in the whole process of sepsis.
Cellular immunity is an important component of sepsis immunity and plays an important role in it. The results confirmed that lymphocyte count, lymphocyte percentage and total T lymphocyte ratio significantly reduce on 1st d sepsis and sepsis shock group came into the ICU, lymphocyte percentage is lower than the normal operation in the control group only on the 3rd d sepsis group came into the ICU and on the 3rd d sepsis shock group came into the ICU their lymphocyte count and lymphocyte percentage are still lower than that of sepsis groups. On the 7th d, most above indicators gradually recovered, but the percentage of total T lymphocytes and the percentage of CD8+ T cells continued to decrease. This study confirmed the decrease of lymphocytopenia appears in the early stage of sepsis, the more T lymphocytopenia reduces, and the more severe the disease was, the more significant the trend was. It suggested that T-cell-mediated immune function was abnormal in the early stage of sepsis and the percentage of total T lymphocytes still decreased in the septic shock group until the 7th d of admission to the ICU, suggesting that the more serious the sepsis condition, the longer the duration of cellular immune suppression. Lymphocyte depletion and apoptosis play important roles in immunosuppression in sepsis. In the study, lymphocyte count and lymphocyte percentage significantly reduced in patients with sepsis and sepsis shock, reflected the lymphocyte depletion. The reduce of total T lymphocyte ratio and the increase of negative stimulation factor expression, such as the increase of the PD-1 expression, reflect the T lymphocyte depletion, suggesting patients with sepsis is in a state of immunosuppression. In this study, sPD-1 levels were significantly different among the four groups of patients on the 1st d of admission to ICU, with high expression in sepsis patients (p<0.05) and even higher in shock patients (p<0.05), suggesting a positive correlation between sPD-1 and the severity of sepsis. This is consistent with the results in previous study [24,25]. With the progression of the disease, the expression of sPD-1 in the septic shock group was still significantly higher than that in the normal operation control group on the 3rd and 7th d (p<0.05). The persistent high expression of sPD-1 indicates that sPD-1 is involved in the immune regulation of sepsis and there is a negative correlation between sPD-1 and the ratio of helper/suppressor T cells on 7th d in the ICU of the septic shock group. Related studies have confirmed that the lower the ratio is, the more serious the immunosuppression is. The high expression of sPD-1 in sepsis patients indicates that they are in the immunosuppression state and the septic shock patients are more serious. Relevant studies have confirmed that the expression of PD-1 on the surface of CD4+ T cells and CD8+ T cells of sepsis patients was still high on the 7th d and its expression was even higher in the death group. In this study, it was found that the percentage of CD8+ T cells in the septic shock group was significantly decreased on the 7th d and sPD-1 was negatively correlated with the ratio of helper/suppressor T cells. sPD-1 may be considered to change the regulation of apoptosis of helper and suppressor T cells with the progression of the disease, but more samples and clinical experiments are still needed for further confirmation. In this study, the patients in the death group had a significant increase in sPD-1 on the 7th d, which may be considered that the immunosuppressive state caused by the continuous high expression of sPD- 1 seriously affects the prognosis of patients.
In the correlation analysis, this study did not find a significant correlation between sPD-1 and immunoglobulin, complement C3, C4, total B lymphocyte ratio, NK cell ratio, etc., but found sPD- 1 and helper/suppressor T cell ratio are negatively correlated. It is considered that PD-1 may affect cellular immune function more directly, but there is no evidence for its effect on humoral immune function and sPD-1 is related to SOFA score, suggesting that sPD-1 is related to the severity of sepsis. In the comparison between the death group and the survival group, there was no difference in sPD-1 between the two groups, suggesting that it cannot alone affect the prognosis of the disease, but the sPD-1 in the death group was significantly increased with the course of the disease (p<0.05). The ROC curve analysis confirms the prognostic value of sPD-1 in patients with sepsis and septic shock. Therefore, dynamically observing the change of sPD- 1 may have a predictive effect on the prognosis of patients.
Acknowledgements:
This work was supported by the Key Project of Dongguan Social Science and Technology Development in 2018 (NO. 2018507150461630).
Conflicts of interest:
The authors report no conflicts of interest.
References
- Almansa R, Tamayo E, Heredia M, Gutierrez S, Ruiz P, Alvarez E, et al. Transcriptomic evidence of impaired immunoglobulin G production in fatal septic shock. J Crit Care 2014;29(2):307-9.
- Brahmamdam P, Inoue S, Unsinger J, Chang KC, McDunn JE, Hotchkiss RS. Delayed administration of anti?PD?1 antibody reverses immune dysfunction and improves survival during sepsis. J Leukoc Biol 2010;88(2):233-40.
- De Backer D, Orbegozo Cortes D, Donadello K, Vincent JL. Pathophysiology of microcirculatory dysfunction and the pathogenesis of septic shock. Virulence 2014;5(1):73-9.
- Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 2011;306(23):2594-605.
- Chang B, Huang T, Wei H, Shen L, Zhu D, He W, et al. The correlation and prognostic value of serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death-ligand 1 (sPD-L1) in patients with hepatocellular carcinoma. Cancer Immunol Immunother 2019;68(3):353-63.
- Chang K, Svabek C, Vazquez-Guillamet C, Sato B, Rasche D, Wilson S, et al. Targeting the programmed cell death 1: programmed cell death ligand 1 pathway reverses T cell exhaustion in patients with sepsis. Crit Care 2014;18(1):1-5.
- El-Gebaly F, Abou-Saif S, Elkadeem M, Helmy A, Abd-Elsalam S, Yousef M, et al. Study of serum soluble programmed death ligand 1 as a prognostic factor in hepatocellular carcinoma in Egyptian patients. Curr Cancer Drug Targets 2019;19(11):896-905.
- Finkelmeier F, Canli Ö, Tal A, Pleli T, Trojan J, Schmidt M, et al. High levels of the soluble programmed death-ligand (sPD-L1) identify hepatocellular carcinoma patients with a poor prognosis. Eur J Cancer 2016;59:152-9.
- Kiyasu J, Miyoshi H, Hirata A, Arakawa F, Ichikawa A, Niino D, et al. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Am J Hematol 2015;126(19):2193-201.
- Leentjens J, Kox M, Koch RM, Preijers F, Joosten LA, van der Hoeven JG, et al. Reversal of immunoparalysis in humans in vivo: a double-blind, placebo-controlled, randomized pilot study. Am J Respir Crit Care Med 2012;186(9):838-45.
- Nielsen C, Ohm-Laursen L, Barington T, Husby S, Lillevang ST. Alternative splice variants of the human PD-1 gene. Cell Immunol 2005;235(2):109-16.
- Perner A, Rhodes A, Venkatesh B, Angus DC, Martin-Loeches I, Preiser JC, et al. Sepsis: frontiers in supportive care, organisation and research. Intensive Care Med 2017;43(4):496-508.
- Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol 2014;35(2):51-60.
- Seymour WC, Liu XV, Iwashyna JT, Brunkhorst MF, Rea DT, Scherag A, et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315(8):762-74.
- Shankar-Hari M, Culshaw N, Post B, Tamayo E, Andaluz-Ojeda D, Bermejo-Martín JF, et al. Endogenous IgG hypogammaglobulinaemia in critically ill adults with sepsis: systematic review and meta-analysis. Intensive Care Med 2015;41(8):1393-401.
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016;315(8):801-10.
- Vajavaara H, Mortensen JB, Leivonen SK, Hansen IM, Ludvigsen M, Holte H, et al. Soluble PD-1 but not PD-L1 levels predict poor outcome in patients with high-risk diffuse large B-cell lymphoma. Cancer 2021;13(3):398.
- Ward NS, Casserly B, Ayala A. The compensatory anti-inflammatory response syndrome (CARS) in critically ill patients. Clin Chest Med 2008;29(4):617-25.
- Van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol 2017;17(7):407-20.
- Wu HP, Chung K, Lin CY, Jiang BY, Chuang DY, Liu YC. Associations of T helper 1, 2, 17 and regulatory T lymphocytes with mortality in severe sepsis. Inflamm Res 2013;62(8):751-63.
- Xie M, Huang X, Ye X, Qian W. Prognostic and clinicopathological significance of PD-1/PD-L1 expression in the tumor microenvironment and neoplastic cells for lymphoma. Int Immunopharmacol 2019;77:105999.
- Giamarellos-Bourboulis EJ, Apostolidou E, Lada M, Perdios I, Gatselis NK, Tsangaris I, et al. Kinetics of circulating immunoglobulin M in sepsis: relationship with final outcome. Crit Care 2013;17(5):1-9.
- Stover CM, McDonald J, Byrne S, Lambert DG, Thompson JP. Properdin levels in human sepsis. Front Immunol 2015;6:24.
- Zhao Y, Jia Y, Li C, Shao R, Fang Y. Predictive value of soluble programmed death-1 for severe sepsis and septic shock during the first week in an intensive care unit. Shock 2019;51(3):289-97.
- Lange A, Sunden-Cullberg J, Magnuson A, Hultgren O. Soluble B and T lymphocyte attenuator correlates to disease severity in sepsis and high levels are associated with an increased risk of mortality. PLoS One 2017;12(1):e0169176.