- *Corresponding Author:
- H. J. Cao
Research Center of Blood Biochemistry and Molecular Biology,
Institute of Blood Transfusion,
Chinese Academy of Medical Sciences and Peking Union Medical College,
Chengdu,
Sichuan 610052
China
E-mail: chjr007@163.com
| This article was originally published in a special issue, “Trending Topics in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(1) Spl Issue “226-234” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Stem cell therapy may be the key to diabetes mellitus. In this study, we systematically retrieved the related studies and conducted this meta-analysis to evaluate the efficacy and safety of stem cell therapy for patients with diabetes mellitus. A systematic articles search was performed for eligible studies published up to April 28, 2021 through the PubMed, Cochrane library, ClinicalTrials.gov and Chinese National Knowledge Infrastructure database. The included articles were screened by using rigorous inclusion and exclusion criteria. All analyses were conducted using Review Manager 5.4. The quality of studies and risk of bias were evaluated. A total of 9 randomized controlled trials were included with a sample size of 326 subjects diagnosed with diabetes mellitus. Stem cells therapy was superior to placebo group in all of outcomes including hemoglobin A1c (mean deviation -0.51, 95 % confidence interval 0.70 to −0.32, p<0.00001), fasting plasma glucose (mean deviation -6.64, 95 % confidence interval -11.58 to -1.71, p=0.008), C-peptide (mean deviation 0.03, 95 % confidence interval 0.02 to 0.04, p<0.00001) and the requirement for insulin (mean deviation -10.34, 95 % confidence interval -16 to -4.67, p<0.00003). There was no significant difference in reported adverse events between stem cell therapy groups and placebo groups (Relative risk 1.77, 95 % confidence interval 0.89 to 3.51, p=0.10). Our results support stem cell therapy is a safe and effective therapeutic modality for diabetes mellitus. The best therapeutic outcome was achieved with bone marrow-mononuclear stem cells and hyperbaric oxygen therapy for type 2 diabetes mellitus, while the poorest outcome was observed with autologous bone marrowmesenchymal stromal cells for type 2 diabetes mellitus.
Keywords
Stem cells therapy, diabetes mellitus, bone marrow-mesenchymal stromal cells, hyperbaric oxygen therapy
According to the World Health Organization, the number of adults with Diabetes Mellitus (DM) has tripled in the past 40 y. In 2014, the global prevalence rate of DM was 8.5 % and the number of adults with DM reached 422 million[1]. The pathogenesis of DM is complex, which is often characterized by insulin resistance or impaired islet secretion function and then involves multiple tissue and organ in the course of disease progressively[2]. There is no known diseasemodifying therapy for DM.
At present, oral hypoglycemic agents and insulin replacement therapy are first-line treatments for DM, which provide the most effective method of blood glucose control for diabetic patients. However, they are not sufficient to reduce diabetes-related autoimmune damage or promote islet cell regeneration, nor can they fundamentally prevent the occurrence of hyperglycemia and related complications in patients with diabetes[3]. As a new choice for the treatment of DM, pancreatic or islet transplantation can achieve the recovery of islet function, but they still face the challenge of donor deficiency and lifelong immunosuppression[4,5]. In recent years, with the clinical treatment of stem cells being used in a variety of major diseases, stem cells have been reported to have achieved good results in the study of diabetes[6,7]. Theoretically, stem cells can be induced to differentiate into insulin-producing beta (β) cells and enhance the survival and function of transplanted islets. It can not only solve the problem of limited islets from suitable donors but also improve the therapeutic effect of islet transplantation in patients with diabetes. Some clinical trials are focusing on the efficacy of stem cell therapy for DM in recent years. To provide clinical references for treating DM, this metaanalysis was performed to evaluate the efficacy and safety of stem cell therapy on patients with DM.
Materials and Methods
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Design and search strategy:
The search included articles in English or Chinese language published in the PubMed, Cochrane library, ClinicalTrials.gov, Chinese National Knowledge Infrastructure database through April 28, 2021. The search was conducted using the following keywords: DM or Diabetes or Type 1 Diabetes Mellitus (T1DM) or Type 2 Diabetes Mellitus (T2DM) or DM and stem cells or cell, stem cells or progenitor cells or mother cells or bone marrow cells or peripheral blood cell or bone marrow and Randomized Controlled Trials (RCTs) or controlled clinical trial. This study was designed and conducted according to the PRISMA reporting guideline[8]. The references to the included articles and reviews were also searched for citations of additional relevant published and unpublished studies.
Criteria for inclusion and exclusion:
Inclusion criteria for the study were a randomized controlled study design that did not require mortality data to ascertain outcome; all subjects were diagnosed with DM; the experimental group was not given intervention other than stem cells therapy under the guarantee of basic medical care; related measure such as Hemoglobin A1c (HbA1c) and Fasting Plasma Glucose (FPG) levels were used to evaluate the pancreatic endocrine function of the experimental group and the control group before and after the intervention. Studies were excluded if the study reported insufficient details to derive the study outcomes; the study had other interventions; full text of the study was not available in the databases; study written in languages other than English and Chinese.
Study outcomes:
We defined efficacy as a significant improvement in pancreatic endocrine function after therapy, including HbA1c, C-peptide level, FPG and insulin requirement. The primary outcome for efficacy was the HbA1c level, which not only can reflect the glycemic control over a long time but also has the characteristic of stability. The secondary outcomes for efficacy were the C-peptide level, FPG and insulin requirement, which are important measures to assess pancreatic endocrine function and the safety outcome of this study was reported adverse events.
Data extraction:
One investigator (Zhangcheng Fei) performed the literature search and screening, and 2 investigators independently performed data extraction. Discrepancies were resolved through discussion between investigators. The extracted data items include study characteristics, including authors, design, country, year of publication; participant characteristics, including age, sex, size, source; type of DM, details of the intervention, treatment duration and all clinical assessment measures.
Risk of bias:
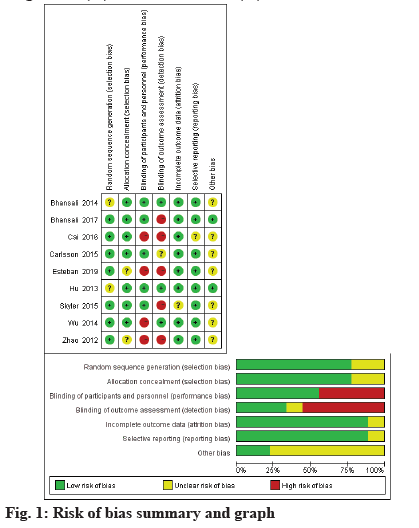
We score the studies that met inclusion criteria according to the Cochrane risk of bias tool, which evaluates the random sequence generation, allocation concealment, blinding of participants, personal and outcome assessment, incomplete outcome data, selective outcome reporting and other biases (fig. 1). The included RCTs were classified as Low risk (L), High risk (H) or Unclear risk (U) in the above items.
Results and Discussion
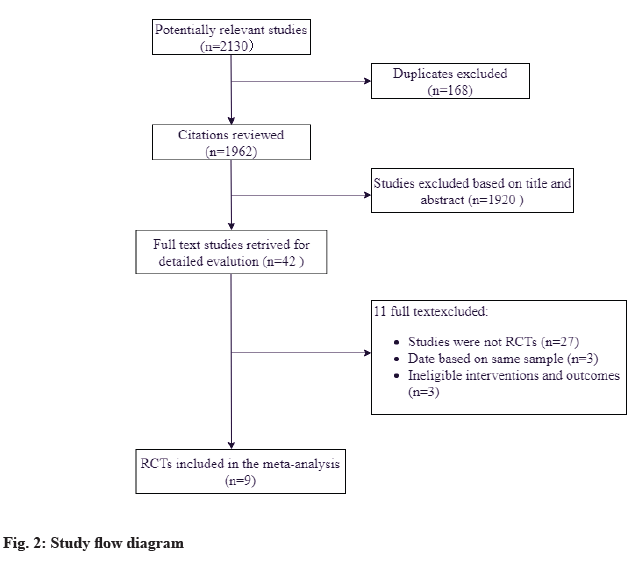
A total of 2130 references were identified from the databases (fig. 2). After excluding duplications and screening of titles and abstracts, the full papers of 42 studies were obtained and assessed for eligibility. According to the inclusion criteria, 9 studies were finally included[2-10]. All of the trials were fully published in peer-reviewed journals between 2012 and 2019 for individuals with DM from United States, India, China, Argentina and Sweden. Out of the 9 trials, stem cell therapy was evaluated in patients with T1DM (4 studies, 106 patients) and T2DM (5 studies, 220 patients). Considering the source of cells, 1 study used Autologous Stem Cell (ASC) (13 patients)[9], 3 studies used Mononuclear Stem Cells (MNCs) (43 patients)[10- 12], 1 study used a combination of different stem cells (21 patients)[13], 3 studies used Mesenchymal Stromal Cells (MSCs) (35 patients)[10,14,15], 1 study used Cord Blood-derived multipotent Stem Cells (CB-SCs) (12 patients)[16], and 1 study used mesenchymal precursor cells (MPCs) (45 patients)[17]. Concrete information of included studies was listed in Table 1. All analyses were conducted using Review Manager 5.4. A fixedeffects model was used for this meta-analysis when there is heterogeneity, or else we will use a randomeffects model. Due to the limitation of data, there was no sensitivity analysis performed.
| Source | Study design | Total (n) | Intervention group Diagnosis Number Body Mass Index (BMI) Age, mean (Standard Deviation (SD) Male (%) Treatment | Control group Diagnosis Number BMI Age, mean (SD) Male (%) Treatment | Dose description | Duration | Efficacy outcome | Risk of bias |
|---|---|---|---|---|---|---|---|---|
| Esteban et al.[9], Argentina | RCT | 23 | T2DM 13 27.0±4.0 59±9 7 (53.8 %) ASC+HOT+standard of care | T2DM 10 23.1±2.5 59±6 5 (50 %) Standard of care | Unknown | 12 mo | Plasma glucose levels HbA1c C-peptide levels The requirement for insulin | L, U, H, H, L, L, U |
| Bhansali et al.[10], India | RCT | 30 | T2DM G1: 10; G2: 10 G1: 28.1 (26.5-31.6); G2: 28.9 (25.0–30.2) G1: 50.5 (36.0–58.0); G2: 44.5 (39.5–49.8) G1: 8 (80 %); G2: 7 (70 %) G1: ABM-MSCs; G2: ABM-MNCs | T2DM 10 25.7 (24.5–28.9) 53.5 (43.3–58.8) 6 (60 %) Placebo | ABM-MSCs: 106/kg; ABM-MNCs:109/person |
12 mo | Plasma glucose levels HbA1c, C-peptide levels, The requirement for insulin | L, L, L, H, L, L, L |
| Carlsson et al.[14], Sweden | RCT | 20 | T1DM 10 22.5±0.9 68±4 5 (50 %) MSCs | T1DM 10 23.3±1.1 78±3 8 (80 %) Standard of care | 2.1–3.6×106/kg | 12 mo | HbA1c, D-peptide levels, The requirement for insulin | L, L, L, U, L, L, U |
| Cai et al.[13], China | RCT | 42 | T1DM 21 21.99±1.78 18.29 9 (42.9) UC-MSC+ABM-MNC | T1DM 21 22.06±2.46 20.38 11 (52.3) Standard of care | 1.1×106/kg UC-MSC, 106.8×106/kg ABM-MNC | 12 mo | Plasma glucose levels, HbA1c, C-peptide levels, The requirement for insulin | L, L, H, H, L, U, U |
| Skyler et al.[17], USA | RCT | 61 | T2DM G1: 15; G2: 15; G3: 15 G1: 34.8±6.5; G2: 34.4±4.7; G3: 32.4±4.5 G1: 57.7±8.2; G2: 55.3±11.4; G3: 57.2±6.6 G1: 10 (66.7); G2: 9 (60.0); G3: 9 (60.0) MPCs | T2DM 16 32.6±6.2 58.7±7.3 12 (75.0) Placebo | G1: 0.3×106/kg; G2: 1.0×106/kg; G3: 2.0×106/kg | 12 w | Plasma glucose levels, HbA1c, C-peptide levels | L, L, L, H, U, L, U |
| Zhao et al.[16], China | RCT | 15 | T1DM G1: 6; G2: 6 severe patients NA G1: 30 (9); G2: 27 (11) G1: 2 (33.3 %); G2: 1 (16.7 %) CB-SCs | T1DM 3 NA 33 (9) 3 (100 %) Placebo | Unknown | 12 w | C-peptide levels | L, U, H, H, L, L, U |
| Hu et al.[15], China | RCT | 29 | T1DM 15 20.9±3.7 17.6±8.7 9 (60 %) WJ-MSCs | T1DM 14 21.3±4.2 18.2±7.9 8 (57 %) Placebo | 2.6×107/kg | 21 mo | NA | U, L, L, L, L, L, L |
| Bhansali et al.[11], India | RCT | 26 | T2DM 13 28.5 (26.3–30.3) 51.0 (46.5–56.0) 9 (69 %) BM-MNCs | T2DM 13 28.9 (26.3–30.3) 54.0 (52.5–55.8) 7 (54 %) Placebo | 2.9×108/kg | 12 mo | Plasma glucose levels, HbA1c, The requirement for insulin | U, L, L, L, L, L, U |
| Wu et al.[12], China | RCT | 80 | T2DM G1: 20; G2: 20; G3: 20 G1: 24.5±1.7; G2: 24.5±2.2; G3: 23.9±3.3 G1: 57.4±5.7; G2: 56.4±5.9; G3: 54.9±6.2 G1: 12 (60 %); G2: 12 (60 %); G3: 10 (50 %) G1: BM-MNCs+HOT; G2: BM-MNCs; G3: HOT | T2DM 20 24.5±2.8 54.9±6.3 11 (55 %) Standard of care | 382.6×107/kg | 12 mo | Plasma glucose levels, HbA1c, C-peptide levels, The requirement for insulin | L, L, H, L, L, L, U |
Table 1: List of Information of Included Studies
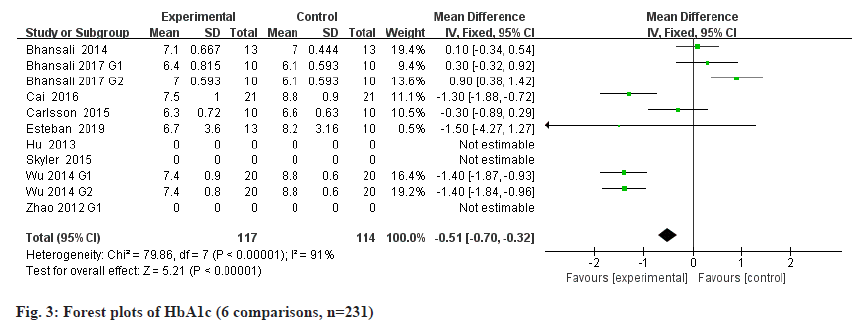
Results of the meta-analysis regarding efficacy outcomes is shown below. The detection of HbA1c is considered to be an important indicator for evaluating the disease control of diabetic patients[18]. The International Diabetes Federation recommended that HbA1c become the gold standard for diabetes monitoring[19], and HbA1c≥6.5 % is considered to be the main diagnostic cut-off point for diabetes clinically. Stem cells therapy was superior to placebo group in all of the available 6 RCTs with 231 patients in terms of HbA1c (fig. 3): Mean Deviation (MD) -0.51, 95 % Confidence Interval (CI) 0.70 to −0.32, p<0.00001; heterogeneity Chi2=79.86, degree of freedom (df)=7, p<0.00001, heterogeneity measure (I2)= 91 %.
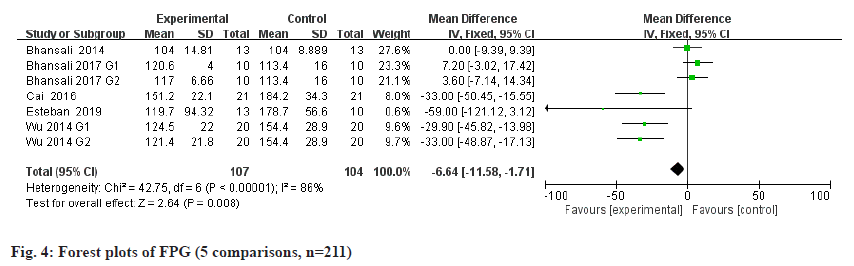
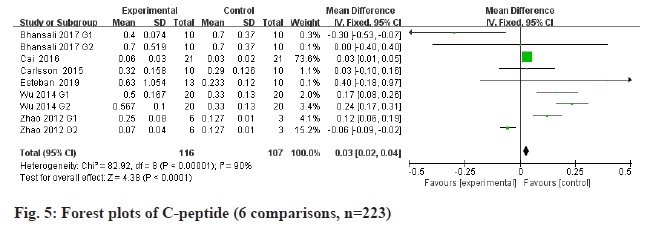
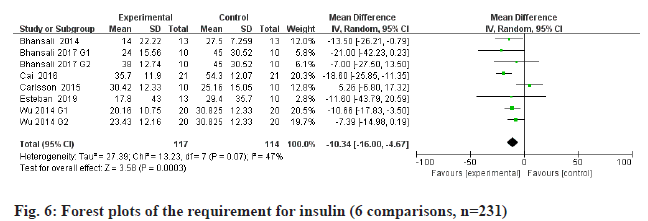
FPG is a metabolic parameter indicating the diagnostic criteria of DM[20]. Information on FPG was available in 5 trials containing 211 patients. The estimated pooled MD for 5 trials shows a highly significant decrease in FPG (fig. 4): MD -6.64, 95 % CI -11.58 to -1.71, p=0.008; heterogeneity Chi2= 42.75, df=6, p<0.00001, I2=86 %). C-peptide is a recognized biomarker of endogenous insulin synthesis, which can reflect the endocrine function of islet β-cells[21]. Stem cells therapy group performed better than placebo group of all the available 6 RCTs with 223 patients in terms of C-peptide (fig. 5): MD 0.03, 95 % CI 0.02 to 0.04, p<0.00001; heterogeneity Chi2=82.92, df=8, p<0.00001, I2= 90 %. The requirement for insulin was significantly lower in the intervention group (fig. 6): MD -10.34, 95 % CI -16 to -4.67, p<0.00003; heterogeneity Chi2=27.39, df=7, p=0.07, I2= 47 %.
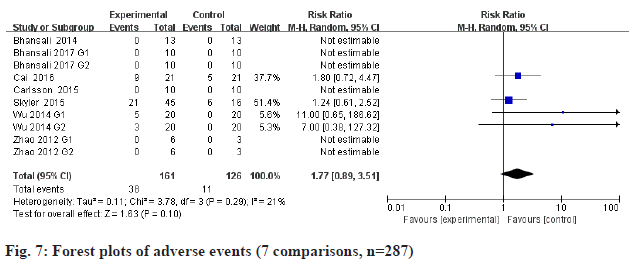
All the included studies were performed safety metaanalysis. There was no significant difference in reported adverse events between stem cell therapy groups and placebo groups (fig. 7): Relative Risk (RR) 1.77, 95 % CI 0.89 to 3.51, p=0.10; heterogeneity Chi2=3.78, df=3, p=0.29, I2=21 %.
DM is a chronic metabolic disease caused by multiple factors, accompanied by a variety of complications. Its drugs are expensive, dependent and unable to fundamentally restore insulin independence. As a progressive disease, DM gradually increases its dependence on exogenous blood glucose control with the development of the natural course of the disease, which eventually leads to complications of multiple tissue lesions[22]. With the deepening of stem cell research in recent years, the restoration of islet function through stem cell transplantation provides a new idea for the treatment of DM.
Although there has been a meta-analysis of stem cell intervention in patients with DM, the quality of evidence of researches was very low because of the inclusion of non-RCTs[23]. This study and meta-analysis only included RCTs that with a higher level of evidence and included some new studies, all non-RCTs such as cohort studies were excluded. It’s found that the difference in all outcomes between stem cell therapy groups and the placebo groups meets statistical significance. For efficacy, this meta-analysis showed significant HbA1c, FGP and insulin requirement reduction and C-peptide increase in DM treated with stem cells compared with the control therapy. Therefore, the results demonstrated that stem cell therapy can improve effectively glycemic control. The best therapeutic outcome was achieved With Bone Marrow-Mononuclear Stem Cells (BM-MNCs)+Hyperbaric Oxygen Therapy (HOT) for T2DM, which may be associated with that HOT mobilizes stem cells by stimulating Nitric Oxide (NO) synthesis[24]. The mechanism of action of stem cell therapy for DM remains unclear, where the underlying mechanism in the improvement in DM may involve the following: Stem cells are highly totipotent or pluripotent, which can differentiate into a variety of tissue cells, replicate and proliferate in vitro and maintain the same population characteristics as parental cells. The use of its directional induction to differentiate into insulin-secreting cells with normal function may reduce or even get rid of patient’s long term dependence on insulin. Stem cells can timely and effectively improve the microenvironment and regulate immune response, which are the key factors in the pathogenesis of DM[25,26]. Animal experiments have shown that MSCs can inhibit the expression of Monocyte Chemotactic Protein 1 (MCP-1) by secreting hepatocyte growth factor, then significantly downregulate the expression of Interleukin 1 beta (IL-1β), Interleukin 6 (IL-6) and Tumour Necrosis Factor alpha (TNF-α), reduce macrophage infiltration, thus decrease blood glucose, alleviate diabetic nephropathy and improve renal function[27]. As an important regulatory pathway to maintain intracellular homeostasis, the imbalance of autophagy-related mechanisms can lead to a decrease in the number and dysfunction of pancreatic β-cells, resulting in a decrease in insulin secretion[28]. And it has been found that tail vein infusion of human umbilical cord MSCs can enhance autophagy of renal tissue cells in DM rats, which can delay renal interstitial fibrosis and improve glucose tolerance in DM rats[29,30]. For safety, there is no significant difference between stem cell therapy and placebo in adverse events. In the patients with DM who received stem cell therapy from the included studies, either no significant adverse reactions occurred or mild adverse reactions spontaneously recovered. So, stem cell therapy is a safe treatment.
Of course, there are several limitations that may affect the results of our meta-analysis. Although the search strategy is strict, we may not be able to include certain studies, such as non-English or non-Chinese and publications that are not in the database we search. The type and dose of stem cell therapy are different, so there is heterogeneity between these studies. The data gathered is limited since this meta-analysis only covers RCTs with high-quality evidence. The original data of some studies are not directly available but need further calculation, which also increases the difficulty of statistics. Finally, we can’t exclude the effect of publication bias and the potential effects caused by some confounders.
In conclusion, it is obvious that stem cell therapy is a safe approach that produces lasting improvement in metabolic control for DM. Larger and longer RCTs are needed in the future to confirm the real clinical potential of stem cell therapy in DM. Moreover, how to improve stem cell transformation into functional cells, how to improve cellular insulin secretion, adjust the host immune response, ensure long-term efficacy stability and safety, and which types of stem cells apply to specific types of DM also need more in-depth research.
Author’s contributions:
Zhangcheng Fei and Renjun Pei contributed equally to this work.
Acknowledgements:
This study was supported by the Scientific Research Project of Sichuan Health Committee (No. 19PJ305), The Science and Technology Project of Sichuan (No. 2019YFS0105) and the Fundamental Research Funds for the Central Universities (No. 3332018126).
Conflict of interests:
The authors declared no conflict of interest.
References
- Roglic G. WHO Global report on diabetes: A summary. Int J Noncommun Dis 2016;1(1):3.
- Chan JC, Lim LL, Wareham NJ, Shaw JE, Orchard TJ, Zhang P, et al. The Lancet commission on diabetes: Using data to transform diabetes care and patient lives. Lancet 2020;396(10267):2019-82.
- Jacobson AM, Braffett BH, Cleary PA, Gubitosi-Klug RA, Larkin ME, DCCT/EDIC research group. The long-term effects of type 1 diabetes treatment and complications on health-related quality of life: A 23-year follow-up of the diabetes control and complications/epidemiology of diabetes interventions and complications cohort. Diabetes Care 2013;36(10):3131-8.
[Crossref] [Google Scholar] [PubMed]
- Bottino R, Knoll MF, Knoll CA, Bertera S, Trucco MM. The future of islet transplantation is now. Front Med 2018;5:202.
- Kandaswamy R, Stock PG, Gustafson SK, Skeans MA, Urban R, Fox A, et al. OPTN/SRTR 2018 annual data report: Pancreas. Am J Transplant 2020;20:131-92.
[Crossref] [Google Scholar] [PubMed]
- Shin L, Peterson DA. Impaired therapeutic capacity of autologous stem cells in a model of type 2 diabetes. Stem Cells Transl Med 2012;1(2):125-35.
[Crossref] [Google Scholar] [PubMed]
- Burt RK, Loh Y, Pearce W, Beohar N, Barr WG, Craig R, et al. Clinical applications of blood-derived and marrow-derived stem cells for nonmalignant diseases. JAMA 2008;299(8):925-36.
[Crossref] [Google Scholar] [PubMed]
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group*. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med 2009;151(4):264-9.
[Crossref] [Google Scholar] [PubMed]
- Estrada EJ, Decima JL, Bortman G, Roberti J, Romero EB, Samaja G, et al. Combination treatment of autologous bone marrow stem cell transplantation and hyperbaric oxygen therapy for type 2 diabetes mellitus: A randomized controlled trial. Cell Transplant 2019;28(12):1632-40.
[Crossref] [Google Scholar] [PubMed]
- Bhansali S, Dutta P, Kumar V, Yadav MK, Jain A, Mudaliar S, et al. Efficacy of autologous bone marrow-derived mesenchymal stem cell and mononuclear cell transplantation in type 2 diabetes mellitus: A randomized, placebo-controlled comparative study. Stem Cells Dev 2017;26(7):471-81.
[Crossref] [Google Scholar] [PubMed]
- Bhansali A, Asokumar P, Walia R, Bhansali S, Gupta V, Jain A, et al. Efficacy and safety of autologous bone marrow-derived stem cell transplantation in patients with type 2 diabetes mellitus: A randomized placebo-controlled study. Cell Transplant 2014;23(9):1075-85.
[Crossref] [Google Scholar] [PubMed]
- Wu Z, Cai J, Chen J, Huang L, Wu W, Luo F, et al. Autologous bone marrow mononuclear cell infusion and hyperbaric oxygen therapy in type 2 diabetes mellitus: An open-label, randomized controlled clinical trial. Cytotherapy 2014;16(2):258-65.
[Crossref] [Google Scholar] [PubMed]
- Cai J, Wu Z, Xu X, Liao L, Chen J, Huang L, et al. Umbilical cord mesenchymal stromal cell with autologous bone marrow cell transplantation in established type 1 diabetes: A pilot randomized controlled open-label clinical study to assess safety and impact on insulin secretion. Diabetes Care 2016;39(1):149-57.
[Crossref] [Google Scholar] [PubMed]
- Carlsson PO, Schwarcz E, Korsgren O, Le Blanc K. Preserved β-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes 2015;64(2):587-92.
[Crossref] [Google Scholar] [PubMed]
- Hu J, Yu X, Wang Z, Wang F, Wang L, Gao H, et al. Long term effects of the implantation of Wharton’s jelly-derived mesenchymal stem cells from the umbilical cord for newly-onset type 1 diabetes mellitus. Endocr J 2013;60(3):347-57.
[Crossref] [Google Scholar] [PubMed]
- Zhao Y, Jiang Z, Zhao T, Ye M, Hu C, Yin Z, et al. Reversal of type 1 diabetes via islet β cell regeneration following immune modulation by cord blood-derived multipotent stem cells. BMC Med 2012;10(1):1-11.
[Crossref] [Google Scholar] [PubMed]
- Skyler JS, Fonseca VA, Segal KR, Rosenstock J. Allogeneic mesenchymal precursor cells in type 2 diabetes: A randomized, placebo-controlled, dose-escalation safety and tolerability pilot study. Diabetes Care 2015;38(9):1742-9.
[Crossref] [Google Scholar] [PubMed]
- Roy S, Jiang JX, Li AF, Kim D. Connexin channel and its role in diabetic retinopathy. Prog Retin Eye Res 2017;61:35-59.
[Crossref] [Google Scholar] [PubMed]
- Dhanoo AS, Cockburn BN, Shah N, Superville R, Hill-Briggs F. Monitoring of international diabetes federation-recommended clinical diabetes indicators in a public health centre in southwest Trinidad. West Indian Med J 2014;63(6):566.
[Crossref] [Google Scholar] [PubMed]
- Tegelberg P, Tervonen T, Knuuttila M, Jokelainen J, Keinänen‐Kiukaanniemi S, Auvinen J, et al. Association of hyperglycaemia with periodontal status: Results of the Northern Finland birth cohort 1966 study. J Clin Periodontol 2021;48(1):25-37.
[Crossref] [Google Scholar] [PubMed]
- Bhatt MP, Lim YC, Ha KS. C-peptide replacement therapy as an emerging strategy for preventing diabetic vasculopathy. Cardiovasc Res 2014;104(2):234-44.
[Crossref] [Google Scholar] [PubMed]
- Raffaele M, Li Volti G, Barbagallo IA, Vanella L. Therapeutic efficacy of stem cells transplantation in diabetes: Role of heme oxygenase. Front Cell Dev Biol 2016;4:80.
[Crossref] [Google Scholar] [PubMed]
- El-Badawy A, El-Badri N. Clinical efficacy of stem cell therapy for diabetes mellitus: A meta-analysis. PLoS One 2016;11(4):e0151938.
[Crossref] [Google Scholar] [PubMed]
- Thom SR. Bhopale VM, Velazquez OC, Goldstein LJ, Thom LH, Buerk DG. Stem cell mobilization by hyperbaric oxygen. Am J Physiol Heart Circ Physiol 2006;290:H1378-86.
[Crossref] [Google Scholar] [PubMed]
- Pirola L, Ferraz JC. Role of pro-and anti-inflammatory phenomena in the physiopathology of type 2 diabetes and obesity. World J Biol Chem 2017;8(2):120.
[Crossref] [Google Scholar] [PubMed]
- Xie Z, Hao H, Tong C, Cheng Y, Liu J, Pang Y, et al. Human umbilical cord-derived mesenchymal stem cells elicit macrophages into an anti-inflammatory phenotype to alleviate insulin resistance in type 2 diabetic rats. Stem Cells 2016;34(3):627-39.
[Crossref] [Google Scholar] [PubMed]
- Lv SS, Liu G, Wang JP, Wang WW, Cheng J, Sun AL, et al. Mesenchymal stem cells transplantation ameliorates glomerular injury in streptozotocin-induced diabetic nephropathy in rats via inhibiting macrophage infiltration. Int Immunopharmacol 2013;17(2):275-82.
[Crossref] [Google Scholar] [PubMed]
- Lee YH, Kim J, Park K, Lee MS. β-cell autophagy: Mechanism and role in β-cell dysfunction. Mol Metab 2019;27:S92-103.
[Crossref] [Google Scholar] [PubMed]
- Tanaka Y, Kume S, Kitada M, Kanasaki K, Uzu T, Maegawa H, et al. Autophagy as a therapeutic target in diabetic nephropathy. Exp Diabetes Res 2012;2012.
[Crossref] [Google Scholar] [PubMed]
- Liu M, Liang K, Zhen J, Zhou M, Wang X, Wang Z, et al. Sirt6 deficiency exacerbates podocyte injury and proteinuria through targeting Notch signaling. Nat Commun 2017;8(1):1-5.
[Crossref] [Google Scholar] [PubMed]