- Corresponding Author:
- S. Paulsamy
PG and Research Department of Botany, Kongunadu Arts and Science College, Coimbatore - 641 029, India
E-mail: paulsami@yahoo.com
| Date of Submission | 20 February 2014 |
| Date of Revision | 19 October 2014 |
| Date of Acceptance | 08 January 2015 |
| Indian J Pharm Sci, 2015;77(1):111-116 |
Abstract
Aim of this study was to develop a TLC and a HPTLC fingerprint profiles for various secondary metabolites of methanol extracts of the stem of the traditional medicinal climber, Solena amplexicaulis. These studies were carried out as per the methods of Harborne and Wagner et al. The profiles of various individual secondary metabolites were made and developed for authentication. The methanol extract of the stem showed the presence of 6 alkaloids, 6 flavonoids, 2 glycosides, 9 saponins and 3 terpenoids. Owing to the presence of rich variety of secondary metabolites, the stem extract of S. amplexicaulis is expected to exhibit therapeutic properties.
Keywords
Solena amplexicaulis, TLC profile, HPTLC profile, bioactive compounds
The current scenario appears to demand for plant drugs throughout the world because of their safety and efficacy [1]. Now a days folklore medicine is being reevaluated by extensive research on different plant species and their therapeutic principles. Chromatographic and spectral fingerprint analysis plays an important role in the quality control of complex herbal medicines [2]. Thin layer chromatography (TLC) is the preliminary step to identify the phytochemical constituents in a sample. High performance thin layer chromatography (HPTLC) can provide an electronic image of the chromatographic fingerprint and a densitogram to detect the presence of marker compounds in a plant sample. Both the methods are efficient, faster, reliable and reproducible [3].
Solena amplexicaulis, commonly called as the creeping cucumber, belongs to the family Cucurbitaceae. The medicinal uses of this species are manyfold [4]. Traditional healers prescribed the tubers of this species as astringent, appetizer, carminative, cardiotonic, digestive, diuretic, expectorant, invigorating, purgative, stimulant, sour and termogenic [5,6]. The whole plant is a potential source of natural antioxidant [7,8], antidiabetic [9] and antibacterial [10] agents. The leaves have good antiinflammatory property, the species is recognized as CNS active, diuretic, febrifuge and hypothermic [6,11]. Crude leaf juice is used to cure jaundice [12]. Raw unripe fruits are eaten to strengthen the body [13]. The decoction of the root is taken orally to cure stomach ache [14], while the seeds are purgative [6].
It is a well known fact that species of medicinal importance contain a rich variety of secondary metabolites, some of which are responsible for the biological activity. To confirm this view, TLC and HPTLC studies were undertaken to explore the various secondary metabolites present in the stems of S. amplexicaulis.
The stems of S. amplexicaulis were collected separately from the thorny scrub jungles of Madukkarai, Coimbatore district, Tamil Nadu, India. The authenticity of the plant was confirmed by comparing with the reference specimens (vide no: CPS 313) preserved at Botanical Survey of India, Southern Circle, Coimbatore. They were washed thoroughly in tap water, shade-dried, homogenized to a fine powder and stored in air tight bottles. Fifty grams of the powdered stem of S. amplexicaulis was extracted with 250 ml methanol at a temperature between 60 and 65° for 24 h using a Soxhlet extractor. The solvent was evaporated in a rotary vacuum evaporator to obtain a viscous semi-solid mass. This semi-dry crude methanol extract was subjected to TLC and HPTLC analysis. TLC and HPTLC studies were carried out using the methods of Harbone [15] and Wagner et al. [16], respectively.
For separating different phytochemical compounds in the methanol extract of the stems of S. amplexicaulis, the extract was spotted manually using a capillary tube on pre coated silica gel G TLC plates (15×5 cm, 3 mm thickness). The spotted plates were developed in different solvent systems to detect a suitable mobile phase as per the method of Wagner et al. [16,17]. After the separation of phytochemical constituents, reagents such as Dragendorff, 10% sulphuric acid in ethanol, 10% sulphuric acid, 5% ferric chloride, Kedde, vanillin phosphoric acid and vanillin sulphuric acid were sprayed to identify the respective compounds. The colours of the spots were noted and Rf values were calculated.
One hundred milligrams of the methanol extract was dissolved in 1 ml of HPTLC grade methanol and centrifuged at 3000 rpm for 5 min. This solution was used as test solution for HPTLC analysis. Different solvent systems were used to develop HPTLC fingerprint profile for different secondary metabolite groups such as alkaloids, flavonoids, glycosides, terpenoids and saponins [15,16,18]. Two microliters of the sample and 3 μl of standard solution were loaded as 5 mm band length separately on pre coated silica gel 60F254 aluminum sheets (3×10 cm) using a Hamilton syringe with the help of Linomat 5 applicator attached to a Camag HPTLC system, which was programmed through WIN CATS software. After the application of spots, the chromatogram was developed in twin trough glass chamber (20×10 cm) pre saturated with respective mobile phase. The air-dried plates were kept in a photo documentation chamber (Camag Reprostar 3) and images were captured at visible light, UV 366 nm and UV 254 nm. The chromatograms were scanned by a densitometer at 405 nm after spraying with respective spray reagents and dried at 100° in a hot air oven. The peak number with its height, area and Rf values of fingerprint data were recorded by WIN CATS (1.3.4 version) software.
The current study was taken up to screen the methanol extract of the stem of S. amplexicaulis for secondary metabolites and develop fingerprints using TLC and HPTLC techniques. Methanol extract of the stem was subjected to TLC in which different mobile phases were tried in order to separate the bioactive compounds like alkaloids, flavonoids, glycosides, terpenoids and saponins (Table 1). The study revealed the development of one orange or brown coloured band for alkaloids in 2 different solvent systems, one yellow or grey coloured band for flavonoids in 2 different solvent compositions and one distinct band for glycosides. In addition, two violet blue coloured spots for saponins in a single solvent system and two blue coloured spots for terpenoids in 2 different compositions were developed by applying respective spraying reagents. Based on the colour, the secondary metabolites were differentiated and Rf values were calculated. The study revealed that relatively high polarity solvents like chloroform, ethyl acetate and methanol were more suited as mobile phases for the separation of bioactive compounds in the stem of S. amplexicaulis. HPTLC profile of methanol extract of the stem was generated in solvent systems of different polarities in order to ascertain the total number of chemical moieties, which will also help in designing the method of isolation and characterization of bioactive compounds (Table 2).
| Mobile phase | Spraying reagent | Colour of thespot/band | Rf | Compound |
|---|---|---|---|---|
| Chloroform-methanol | ||||
| [3:1.3] | Dragendorff reagent/10% | Orange/Brown | 0.21 | Alkaloids |
| [3:2] | H2SO4 in ethanol reagent | 0.25 | ||
| Ethyl acetate-methanol-water-glacial acetic acid | 10% H2SO4/5% ferric | Yellow/Grey | Flavonoids | |
| [1.35:0.5:0.5:0.05] | chloride solution | 0.91 | ||
| Ethyl acetate-methanol-water-toluene | ||||
| [1.4:0.5:0.5:0.05] | 0.92 | |||
| Ethylacetate-methanol | Kedde reagent | Distinct band | Glycosides | |
| [1.3:0.5] | formation | 0.38 | ||
| Chloroform-methanol | Vanillin H2SO4 reagent | Violet blue | 0.69; 0.55 | Saponins |
| [1.2:0.2] | (2 spots) | |||
| Petroleum ether-ethyl acetate | Vanillin phosphoric acid | Blue | 0.39; 0.58 | Terpenoids |
| [2:0.5] | reagent | (2 spots) | ||
| Hexane-ethyl acetate | 0.55; 0.72 | |||
| [1.5:0.5] | (2 spots) |
Table 1: Tlc screening of phytochemicals in methanol extract of the stem of solena Amplexicaulis
| Name of thecompound | Mobile phase | Spray reagent | Colour of the spot/band | |
|---|---|---|---|---|
| Visibile light | UV (366nm) | |||
| Alkaloids | Ethyl acetate-methanol-water | Dragendorff’s reagent followed by 10% | Yellow, | Nil |
| (10:1.35:1) | ethanolicsulphuric acid reagent | orange-yellow | ||
| Flavonoids | Toluene-acetone-formic acid | 1% Ethanolicaluminium chloride reagent | Nil | Yellow, |
| (4.5 : 4.5 : 1) | yellowish blue | |||
| Glycosides | Ethyl acetate-ethanol-water | Anisaldehydesulphuric acid reagent | Pinkish violet | Nil |
| (8 : 2 : 1.2) | ||||
| Saponins | Chloroform-glacial acetic acid-methanol-water Anisaldehydesulphuric acid reagent | Blue, yellow, | Nil | |
| (6.4 : 3.2 : 1.2 : 0.8) | green, violet | |||
| Terpenoids | n-Hexane-ethyl acetate | Anisaldehydesulphuric acid reagent | Blue, bluish | Nil |
| (7.2 : 2.9) | violet | |||
Table 2: Various secondary metabolites observed in hptlc of methanol extract of the stem of solena amplexicaulis
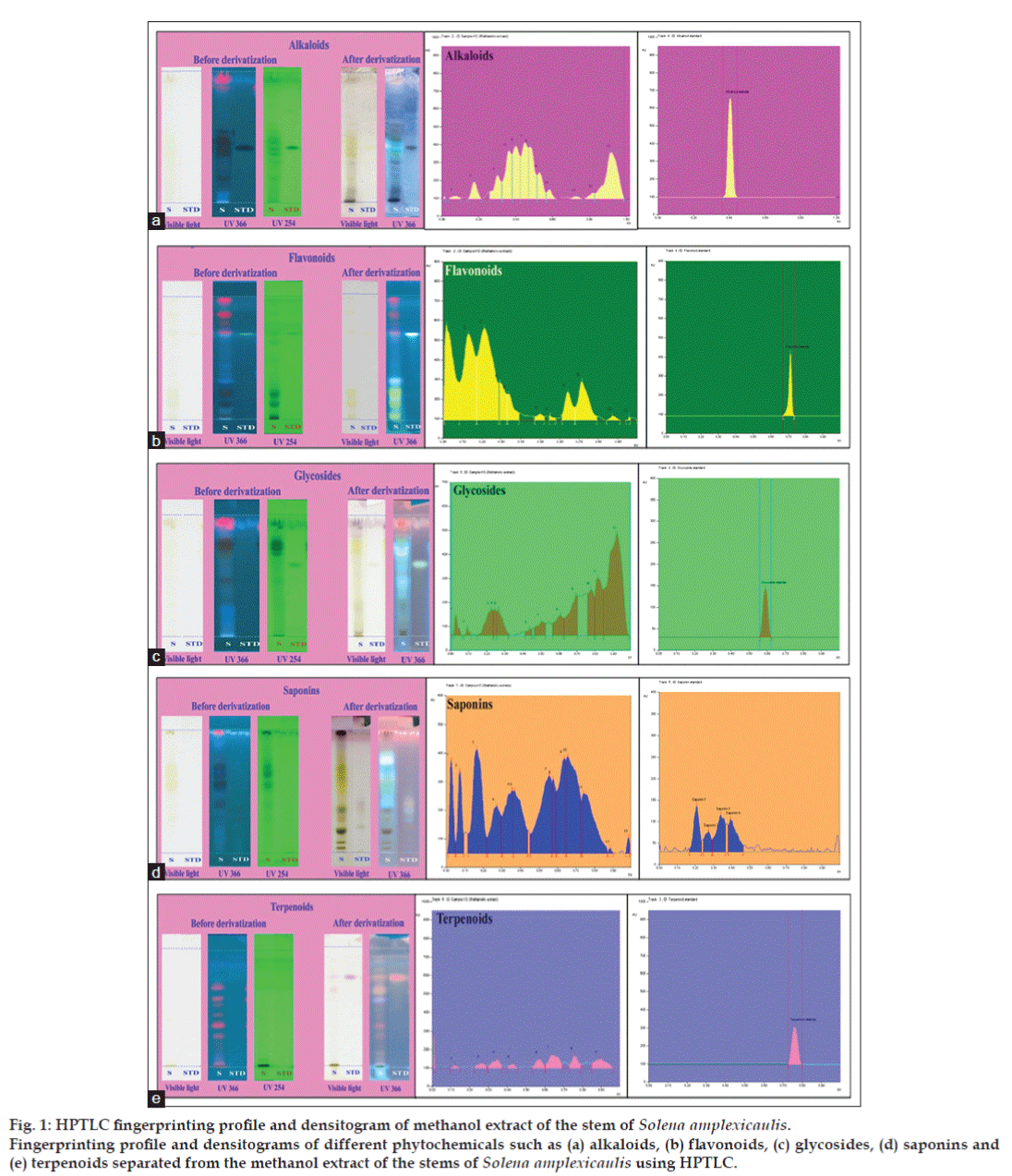
HPTLC alkaloid profile of methanol extract of the S. amplexicaulis stem was presented in Tables 2 and 3 and fig. 1a. Bright orange colour zone at visible light mode was observed in the chromatogram after derivatization, which confirmed the presence of alkaloids in the sample as in the standard. Thirteen compounds were separated and of which 6 were alkaloids at the Rf in the range of 0.18-0.59. The highest peak area was 11283.1 AU and that of the lower one was 1424.5 AU, which were observed at Rf of 0.40 and 0.59, respectively. It is not uncommon to find alkaloids since more than 10 000 different alkaloids have been identified in species from over 300 plant families [19].
Figure 1: HPTLC fingerprinting profile and densitogram of methanol extract of the stem of Solena amplexicaulis.
Fingerprinting profile and densitograms of different phytochemicals such as (a) alkaloids, (b) flavonoids, (c) glycosides, (d) saponins and (e) terpenoids separated from the methanol extract of the stems of Solena amplexicaulis using HPTLC.
| Peak | Rf | Height (mm) | Area (AU) | Assigned substance |
|---|---|---|---|---|
| 1 | 0.01 | 15.5 | 82.2 | Unknown |
| 2 | 0.07 | 15.3 | 539.0 | Unknown |
| 3 | 0.18 | 95.0 | 2872.1 | Alkaloid 1 |
| 4 | 0.30 | 131.3 | 5068.2 | Alkaloid 2 |
| 5 | 0.36 | 267.4 | 9095.2 | Alkaloid 3 |
| 6 | 0.40 | 295.2 | 11283.1 | Alkaloid 4 |
| 7 | 0.45 | 315.2 | 10895.2 | Unknown |
| 8 | 0.48 | 289.5 | 8692.7 | Alkaloid 5 |
| 9 | 0.53 | 146.8 | 4646.9 | Unknown |
| 10 | 0.59 | 52.2 | 1424.5 | Alkaloid 6 |
| 11 | 0.73 | 12.7 | 431.4 | Unknown |
| 12 | 0.82 | 38.3 | 1000.6 | Unknown |
| 13 | 0.92 | 268.3 | 18796.9 | Unknown |
| 1 | 0.30 | 353.9 | 10774.5 | Alkaloid standard |
Table 3: Alkaloids in the hptlc profile of the methanol extract of the stem of solena amplexicaulis
Six different types of flavonoids were observed out of 11 bands in the methanol extract of S. amplexicaulis. The Rf values determined for the flavonoids were in the range of 0.02-0.64 (Tables 2 and 4 and fig. 1b). The suitable solvent system evaluated was toluene-acetone-formic acid (4.5:4.5:1). Yellow or yellowish blue coloured fluorescence zone at UV 366 nm mode confirmed the presence of flavonoids in the sample and standard. The highest peak area was 32643.4 AU and that of the lowest one was 427.9 AU, which were observed at Rf of 0.21 and 0.55, respectively. More than 6000 different flavonoids have been identified to occur and many of them are responsible for the attractive colors of flowers, fruits and leaves [20]. Biological activities exhibited by some of these flavonoids have resulted in intensive research efforts to understand the impact of these compounds on human health [21].
| Peak | Rf | Height (mm) | Area (AU) | Assigned substance |
|---|---|---|---|---|
| 1 | 0.02 | 485.4 | 20704.0 | Flavonoid 1 |
| 2 | 0.13 | 440.6 | 24890.9 | Flavonoid 2 |
| 3 | 0.21 | 468.1 | 32643.4 | Flavonoid 3 |
| 4 | 0.29 | 191.1 | 4995.5 | Flavonoid 4 |
| 5 | 0.34 | 134.6 | 4114.9 | Unknown |
| 6 | 0.50 | 31.1 | 1045.7 | Unknown |
| 7 | 0.55 | 22.1 | 427.9 | Flavonoid 5 |
| 8 | 0.64 | 144.1 | 5201.9 | Flavonoid 6 |
| 9 | 0.71 | 199.0 | 9599.3 | Unknown |
| 10 | 0.87 | 21.7 | 639.7 | Unknown |
| 11 | 0.96 | 16.9 | 139.6 | Unknown |
| 1 | 0.71 | 364.8 | 7412.5 | Flavonoid standard |
Table 4: Flavonoids in the hptlc profile of the methanol extract of the stem of solena amplexicaulis
After derivatization, the pinkish violet colour confirmed the presence of glycosides in the given samples and standard. The sample revealed 12 spots and among them only 2 bands were for glycosides in the Rf level of 0.69 and 0.82 (Tables 2 and 5 and fig. 1c). Reliable solvent system to observe the above separation is ethylacetate: ethanol: water (8:2:1.2). Many plant glycosides are reported to have useful medicinal properties. In animals and humans, poisons are often eliminated from the body as glycosides [22].
| Peak | Rf | Height (mm) | Area (AU) | Assigned substance |
|---|---|---|---|---|
| 1 | 0.03 | 84.7 | 1526.9 | Unknown |
| 2 | 0.09 | 32.2 | 484.5 | Unknown |
| 3 | 0.23 | 108.8 | 4099.3 | Unknown |
| 4 | 0.25 | 110.9 | 1697.0 | Unknown |
| 5 | 0.27 | 109.0 | 3185.4 | Unknown |
| 6 | 0.44 | 29.0 | 650.0 | Unknown |
| 7 | 0.50 | 58.7 | 2437.2 | Unknown |
| 8 | 0.61 | 83.3 | 3547.7 | Unknown |
| 9 | 0.69 | 166.5 | 7167.7 | Glycoside 1 |
| 10 | 0.78 | 190.9 | 6110.6 | Unknown |
| 11 | 0.82 | 240.9 | 8708.6 | Glycoside 2 |
| 12 | 0.92 | 423.5 | 29759.9 | Unknown |
| 1 | 0.59 | 122.0 | 3589.4 | Glycoside standard |
Table 5: Glycosides in the hptlc profile of the methanol extract of the stem of solena amplexicaulis
The HPTLC chromatogram can be best observed under daylight, UV 254 nm and 366 nm before and after derivatization. Nine bands of different types of saponins were seen before derivatization at visible mode. Due to the great variability of their structures, saponins display antitumorigenic effects by activating a variety of antitumor pathways [23]. The highest peak area, 19052.2 AU and the lowest peak area, 5158.5AU were observed at Rf of 0.03 and 0.66, respectively. Best solvent system to be observed for the above separation is chloroform: glacial acetic acid: methanol: water (6.4:3.2:1.2:0.8) (Tables 2 and 6, fig. 1d).
| Peak | Rf | Height (mm) | Area (AU) | Assigned substance |
|---|---|---|---|---|
| 1 | 0.03 | 323.3 | 5158.5 | Saponin 1 |
| 2 | 0.07 | 284.5 | 5571.6 | Saponin 2 |
| 3 | 0.17 | 363.8 | 16539.7 | Saponin 3 |
| 4 | 0.27 | 165.4 | 8169.9 | Saponin 4 |
| 5 | 0.35 | 219.1 | 8434.2 | Saponin 5 |
| 6 | 0.37 | 219.1 | 10054.1 | Saponin 6 |
| 7 | 0.56 | 270.5 | 16269.4 | Saponin 7 |
| 8 | 0.58 | 259.4 | 4324.4 | Unknown |
| 9 | 0.64 | 332.0 | 11640.5 | Saponin 8 |
| 10 | 0.66 | 337.7 | 19052.2 | Saponin 9 |
| 11 | 0.74 | 207.9 | 12969.1 | Unknown |
| 12 | 0.89 | 16.3 | 219.9 | Unknown |
| 13 | 0.98 | 54.3 | 584.4 | Unknown |
| 1 | 0.21 | 105.7 | 327.3 | Saponin standard 1 |
| 2 | 0.28 | 46.1 | 1464.1 | Saponin standard 2 |
| 3 | 0.34 | 84.3 | 3715.2 | Saponin standard 3 |
| 4 | 0.39 | 74.4 | 3075.9 | Saponin standard 4 |
Table 6: Saponins in the hptlc profile of the methanol extract of the stem of solena amplexicaulis
Terpenoids can be observed under daylight, 254 nm and 366 nm before derivatization and after derivatization, blue, bluish violet colour under visible light confirmed the presence of terpenoids in the sample and standard. Three different terpenoids were separated by visualising them in the Rf range of 0.57-0.76 (Tables 2 and 7 and fig. 1e). The highest and lowest peak areas, 3727.1 AU and 1782.0 AU were observed at the Rf of 0.64 and 0.57, respectively. Suitable solvent system determined was n-hexane: ethylacetate (7.2:2.9). More than 40 000 individual terpenoids are known to exist in nature with new compounds being discovered every year [24]. A large number of terpenoids exhibited cytotoxicity against a variety of tumor cell lines as well as anticancer efficacy in preclinical animal models [25,26]. Based on the results obtained in the study, we conclude that the methanol extract of stems of S. amplexicaulis has considerable amount of secondary metabolites, some of which could be developed as pharmacotherapeutic agent in future. The fingerprints developed in this study are likely to aid in the quality control and standardization of herbal formulations containing this plant.
| Peak | Rf | Height (mm) | Area (AU) | Assigned substance |
|---|---|---|---|---|
| 1 | 0.01 | 181.6 | 652.1 | Unknown |
| 2 | 0.12 | 16.0 | 325.8 | Unknown |
| 3 | 0.27 | 27.8 | 969.1 | Unknown |
| 4 | 0.35 | 48.9 | 1913.8 | Unknown |
| 5 | 0.42 | 23.2 | 652.6 | Unknown |
| 6 | 0.57 | 50.2 | 1782.0 | Terpenoid 1 |
| 7 | 0.64 | 74.0 | 3727.1 | Terpenoid 2 |
| 8 | 0.76 | 69.6 | 2319.7 | Terpenoid 3 |
| 9 | 0.89 | 51.5 | 3128.3 | Unknown |
| 1 | 0.71 | 143.0 | 4328.0 | Terpenoid standard |
Table 7: Terpenoids in the hptlc profile of the methanol extract of the stem of solena amplexicaulis
Acknowledgments
The authors graciously acknowledge the financial support given by University Grants Commission, New Delhi (Grant No. F. 41?415/2012(SR) to carry out the work.
References
- Ahmed MF, Rao AS. Comparative hepatoprotective activities of selected Indian medicinal plants. Global J Med Res 2013;13:15-21.
- Gong F, Wang BT, Chau FT, Liang YZ. HPLC data preprocessing for chromatographic fingerprint of herbal medicine with chemometricapproaches. Anal Lett 2005;38:2475-92.
- Moffat CA. In: Clarke’s analysis of drugs and poisons. London: Pharmaceutical Press; 2001. p. 392.
- Yuan G, Wahlqvist ML, He G, Yang M, Li D. Natural products and antiinflammatory activity. Asia Pac J ClinNutr 2006;15:143-52.
- Kritchevsky D. Fiber, lipids and atherosclerosis. Am J ClinNutr 1978;31S:65-74.
- Dhananjay J, Deshpande. A hand book of medicinal herbs. Jodhpur, India: Agrobios; 2006, p. 325-6.
- Venkateshwaralu E, Raghuram Reddy A, Goverdhan P, Swapna Rani K, Jayapal Reddy G. In vitro and in vivo antioxidant activity of methanolic extract of Solenaamplexicaulis (whole plant). Int J Pharm Bio Sci 2011;1:522-33.
- Karthika K, Paulsamy S, Jamuna S. Evaluation of in vitro antioxidant potential of methanolic leaf and stem extracts of Solenaamplexicaulis(Lam.) Gandhi. J Chem Pharm Res 2012;4:3254-8.
- Pullaiah T, Murthy KS, Goud PS, Kumar TD, Vijayakumar R. Medicinal plants used by the tribals of Nallamalais, Eastern Ghats of India. J Trop Med Plant 2003;4:237-44.
- Karthika K, Paulsamy S. Antibacterial potential of traditional plant species Solenaamplexicaulis (Lam.) Gandhi. against certain human pathogens. Asian J Pharm Clin Res 2012;5(Suppl 4):255-7.
- Arun CH, Satheesh Kumar R, Srinu S, LalBabu G, Raghavendra Kumar G, Amos Babu J. Antiinflammatory activity of aqueous extract of leaves of Solenaamplexicaulis.Int J Res Pharm Biomed Sci 2011;2:1617-9.
- Mohammed R, Paritosh C, Alok Kumar P, Dilruba N, Rasheda A, Farhana J, et al. A survey of preventive medicinal plants used by the Chakma residents of Hatimara (south) village of Rangamati district, Bangladesh. Am Eurasian J Sustain Agric 2011;5:92-6.
- Jeyaprakash K, Ayyanar M, Geetha KN, Sekar T. Traditional uses of medicinal plants among the tribal people in Theni District (Western Ghats), Southern India. Asian Pac J Trop Biomed 2011;1:S20-5.
- Ghorbani A, Langenberger G, Feng L, Sauerborn J. Ethnobotanical study of medicinal plants utilised by Hani ethnicity in Naban River Watershed National Nature Reserve, Yunnan, China. J Ethnopharmacol 2011;134:651-67.
- Harborne JB. Phytochemical methods. 3rd ed. London: Chapman and Hall; 1998.
- Wagner H, Baldt S, Zgainski EM. Plant drug analysis. New York,: Springer; 1996. p. 230-1.
- Wagner H, Bladt S, Zgainski EM. Plant drug analysis – A thin layer chromatography atlas. Berlin: Springer-Verlag; 1984.
- Reich E, Schibili A. High performance thin layer chromatography for the analysis of medicinal plants. New York: Thieme; 2007. p. 224-40.
- Raffauf RF. Plant Alkaloids: A guide to their discovery and distribution. New York: Hawkworth Press Inc.; 1996.
- Ferrer JL, Austin MB, Stewart C Jr, Noel JP. Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. Plant PhysiolBioch 2008;46:356-70.
- Falcone Ferreyra ML, Rius SP, Casati P. Flavonoids: Biosynthesis, biological functions and biotechnological application. Front Plant Sci 2012;3:1-15.
- Kren V, Martínková L. Glycosides in Medicine: The role of glycosidic residue in biological activity. Curr Med Chem 2001;8:1303-28.
- Man S, Gao W, Zhang Y, Huang L, Liu C. Chemical study and medical application of saponins as anticancer agents. Fitoterapia 2010;81:703-14.
- Tsuyoshi G, Nobuyuki T, Shizuka H, Teruo K. Various terpenoids derived from herbal and dietary plants function as PPAR Modulators and regulate carbohydrate and lipid metabolism. Egypt: Hindawi Publishing Corporation; 2010. p. 1-9.
- Nassar Z, Aisha A, Abdul Majid A. The pharmacological properties of terpenoids from SandoricumKoetjape. ?Webmed Central Complement Med 2010;1:WMC001311
- Thoppil RJ, Bishayee A. Terpenoids as potential chemopreventive and therapeutic agents in liver cancer. World J Hepatol 2011;3:228-49.