- *Corresponding Author:
- Liyang Sun
Department of Neurosurgery, Lanxi People's Hospital, Lanxi, Jinhua, Zhejiang 321100, People's Republic of China
E-mail: lxsly9001@163.com
| Date of Received | 27 September 2021 |
| Date of Revision | 08 October 2022 |
| Date of Acceptance | 13 June 2023 |
| Indian J Pharm Sci 2023;85(3):822-828 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Total flavones of Selaginella uncinata (Desv.) spring has inhibitory effects on cancer progression. The study aims to analyze the effects of total flavones of Selaginella uncinata on breast cancer cell tumor properties and the possible mechanism. MDA-MB-231 cells were treated with total flavones of Selaginella uncinata or transfected with anti-microRNA-negative control or anti-microRNA-1269. In addition, microRNA-negative control or microRNA-1269 mimics-transfected MDA-MB-231 cells were exposed to total flavones of Selaginella uncinata. Cell proliferation and apoptosis were investigated via cell counting kit-8, cell colony formation or flow cytometry analysis. MicroRNA-1269, cleaved caspase-3 or cleaved caspase-9 expression was determined through quantitative polymerase chain reaction or Western blotting. After total flavones of Selaginella uncinata treatment, the cell proliferation inhibition rate, cell apoptosis rate, cleaved caspase-3 and cleaved caspase-9 protein levels were increased, the number of cell colonies was decreased, and microRNA-1269 was downregulated in a dose-dependent way. Relative to MCF-10A cells, microRNA-1269 expression was upregulated in MDA-MB-231 cells. After transfection of anti-microRNA-1269, cell apoptosis rate, cleaved caspase-3 and cleaved caspase-9 protein levels and the cell proliferation inhibition rate were increased, but cell colony-forming ability was decreased. After treatment of microRNA-1269 mimics and total flavones of Selaginella uncinata, the cell proliferation inhibition rate, cell apoptosis rate, cleaved caspase-3 and cleaved caspase-9 protein levels were reduced and cell colony-forming ability was promoted. Total flavones of Selaginella uncinata can inhibit the proliferation and cell colony-forming ability and induce apoptosis of breast cancer cells via negatively modulating microRNA-1269.
Keywords
Breast cancer, flavones, Selaginella uncinata, microRNA-1269, cell proliferation, apoptosis
Breast cancer is a heterogeneous tumor that is commonly diagnosed in women and its incidence is increasing in China. Estrogen level, gene mutation and other factors are closely related to the incidence[1]. Currently, surgery, radiotherapy, chemotherapy and other means are often used to treat breast cancer, but the toxic and side effects on breast cancer patients are relatively large and the prognosis of patients is poor[2,3]. Chinese traditional medicine has anti- inflammatory and anti-tumor effects and plays an anti-breast cancer role in many ways[4-6]. Selaginella uncinata (S. uncinata) (Desv.) spring belongs to Selaginellaceae family and has the effect of clearing heat and detoxifying[7]. Total Flavones of S. uncinata (Desv.) Spring (TFS) can inhibit lung cancer cell growth and cell cycle progression[8]. But, the effects of the extract of TFS on breast cancer cell biological behaviors are unknown. MicroRNA (miR-1269) is up-regulated in esophageal squamous cell carcinoma tissues, and promotes cancer cell proliferation, migration and invasion[9]. However, no study has shown whether miR-1269 is a potential target for TFS-mediated treatment for breast cancer. Therefore, this study is designed to demonstrate whether TFS can affect breast cancer cell tumor properties through miR-1269.
Materials and Methods
Reagents:
S. uncinata (Desv.) spring was purchased from Sanyuan Tianyu Biological Products Co., Ltd. Shanghai Yubo Biotechnology Co., Ltd. provided human normal mammary epithelial cells MCF-10A and MDA-MB-231 cells. Thermo Fisher Biotech (Waltham, Massachusetts and United States of America (USA)) supplied RNA isolation reagents as well as Lipofectamine 2000. Fluorescence quantitative Polymerase Chain Reaction (PCR) reagents and complementary Deoxyribonucleic Acid (cDNA) synthesis reagents were provided by Tiangen Biotech (Beijing, China). Ribobio Co., Ltd. (Guangzhou, China) supplied anti-miR-NC, anti-miR-1269, miR-NC and miR-1269. Beyotime Biology (Shanghai, China) provided Cell Counting Kit-8 (CCK-8) reagent and cell apoptosis detection kit. The primary antibodies against caspase-3 and caspase-9 were provided by Amyjet (Wuhan, China). Abcam (Cambridge, Massachusetts, USA) supplied the primary antibody specific to Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) and the secondary antibody.
Method:
Preparation of TFS: Preparation of TFS was performed in accordance with previous methods[10]. 1000 g of S. uncinata (Desv.) spring was ground into powder, filtered through 60-mesh sieves and mixed with 60 % ethanol. After heating the mixture for 20 min, filtrate was collected and purified after decompression, evaporation and drying. The extracts were eluted with 70 % ethanol, concentrated and dried to obtain the extracts. After that, methanol was added to the extracts, and the peak wavelength of 150 nm was detected by enzyme-linked immune detector with rutin as the control substance. The absorption spectrum of the extracts was similar to that of rutin. Standard curve was made according to the value of rutin and then the content of TFS was calculated. Through calculation, we determined that the total content of TFS was 2.66 mg/g. TFS was dissolved in Dimethyl Sulfoxide (DMSO) and then added into medium to prepare 1 mg/ml TFS. According to the experimental requirements, the TFS was diluted to 5, 15 and 25 μg/ml.
Cell treatments: MDA-MB-231 cells were cultured for 24 h in medium containing TFS with different concentrations (5, 15 and 25 μg/ ml), which were classified as the TFS-L group, TFS-M group and TFS-H group, respectively. At the same time, cells cultured in medium containing DMSO were regarded as the control group. To determine miR-1269 mediated influence on breast cancer cell proliferative and apoptotic abilities, we knocked down miR-1269 by transfecting miR-1269 inhibitors and its control into MDA- MB-231 cells, which were named as anti-miR-NC group and anti-miR-1269 group, respectively. To determine whether TFS affected breast cancer cell proliferative and apoptotic abilities by regulating miR-1269, the study performed transfection of miR-1269 mimics into MDA-MB-231 cells for 48 h, followed by culturing with medium containing 25 μg/ml TFS for 2 d, classified as the TFS+miR-NC group and the TFS+miR-1269 group, respectively.
Cell proliferation inhibition rate: Breast cancer cells from each group were collected and inoculated in 35 mm petri dishes (2×103 cells/well). Cells were cultured with CCK-8 solution in an incubator with 5 % Carbon dioxide (CO2). Samples were detected by microplate reader. Cell proliferation inhibition rate=((Control group Optical Density (OD)–experimental group OD)/(control group OD–blank group OD)×100 %)
Cell colony formation assay: 0.25 % trypsin was used for digestion and the supernatant was discarded following centrifugation at 3000 r/min for 6 min. Phosphate buffer solution was used to wash the cell precipitation and 500 μl binding buffer was added. Annexin V-Fluorescein Isothiocyante (FITC) and prodium iodide were added to each well respectively. FACS Calibur flow cytometer was utilized to analyze cells.
MiR-1269 expression analysis by quantitative Reverse Transcription PCR (qRT-PCR):
The MCF-10A cells and MD-MB-231 cells were taken out and placed into Eppendorf (EP) tubes and 1 ml Trizol reagent was added to lyse samples. The cells were centrifuged at 12 000 r/min. Chloroform and isopropyl alcohol was added to each EP tube respectively and supernatant was discarded after centrifugation. The isolated Ribonucleic Acid (RNA) was then reversely transcribed to cDNA, referring to the guidebooks. qRT-PCR samples were prepared according to the following reaction system including 10 μl SYBR Mixture, 0.8 μl positive as well as negative primers, 1 μl cDNA, and 7.4 μl double-distilled Water (ddH2O). The samples were reacted on ABI Step One Plus qRT-PCR and miR-1269 expression was finally analyzed.
Western blotting analysis:
MDA-MB-231 cell samples of each group were added with 500 μl Radioimmunoprecipitation Assay (RIPA) buffer. 5×Sodium Dodecyl Sulfate (SDS) loading buffers were mixed with samples and boiled in hot water. Some protein samples were used for protein concentration detection via the Bicinchoninic Acid (BCA) method. After analysis of sample contents, 40 μg protein was used for SDS-Polyacrylamide Gel (SDS-PAGE) and protein bands were subjected to Polyvinylidene Difluoride (PVDF) membrane transfer and blocked at room temperature. The membranes were incubated with anti-cleaved caspase-3 (1:1000), anti-cleaved caspase-9 (1:1000), and anti-GAPDH (1:3000) for 24 h and secondary antibodies (1:5000) for 1 h. After Enhanced Chemiluminescence (ECL) was uniformly added to the membranes, the membranes were exposed and developed in the darkroom and the gray values of all bands were assessed by Image J software.
Statistical analysis:
Statistical Package for the Social Sciences (SPSS) 21.0 was used to analyze data and measurement data were expressed as (x̄ ±s) t test and one-way analysis of variance were used for comparison p<0.05indicated statistically significant.
Results and Discussion
As shown in Table 1, cell proliferation inhibition rates in TFS-L, TFS-M and TFS-H groups were increased after TFS treatment in dose-dependent manners (p<0.05), while TFS treatment reduced the number of cell colonies (p<0.05).
| Group | Cell proliferation inhibition rate (%) | Number of cell colonies |
|---|---|---|
| Control | 0.00±0.00 | 111.72±9.50 |
| TFS-L | 25.31±2.11* | 91.18±7.64* |
| TFS-M | 43.61±4.29*# | 73.49±6.72*# |
| TFS-H | 64.23±5.52*#& | 56.08±4.57*#& |
| F | 503.094 | 95.414 |
| p | 0 | 0 |
Note: *p<0.05, #p<0.05 and &p<0.05 relative to control group, TFS-L group and TFS-M group, respectively
Table 1: TFS Mediated effect on MDA-MB-231 Cell Proliferation (x̄±s, n=9)
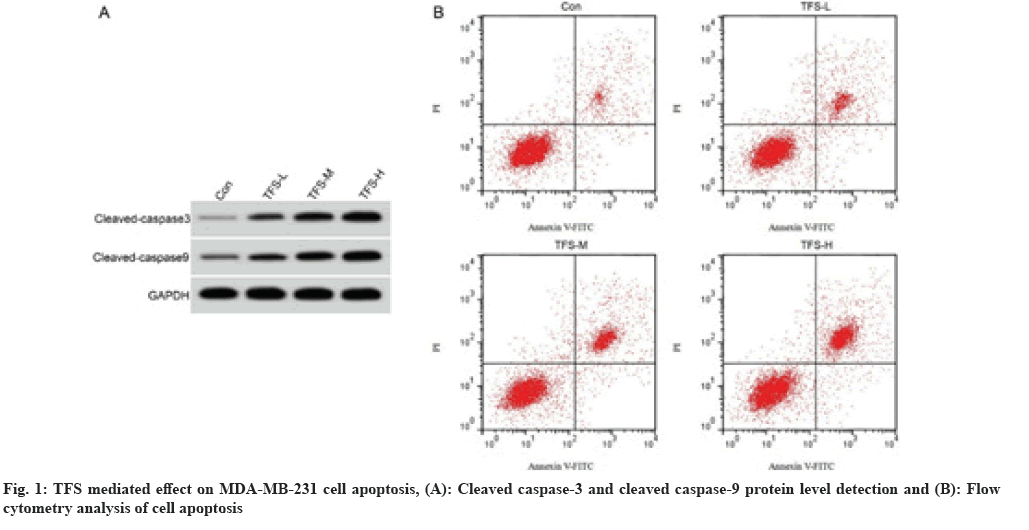
As shown in fig. 1 and Table 2, the cell apoptosis rate and cleaved caspase-3 and cleaved caspase-9 protein levels in TFS-L, TFS-M and TFS-H groups were increased after TFS treatment in dose-dependent manner (p<0.05).
| Group | Apoptotic rate (%) | Cleaved caspase-3 protein expression | Cleaved caspase-9 protein expression |
|---|---|---|---|
| Control | 6.75±0.66 | 0.16±0.02 | 0.23±0.02 |
| TFS-L | 12.02±1.10* | 0.29±0.02* | 0.39±0.03* |
| TFS-M | 18.32±1.21*# | 0.41±0.03*# | 0.53±0.04*# |
| TFS-H | 4 | 0.56±0.05*#& | 0.68±0.05*#& |
| F | 388.945 | 249.429 | 246.833 |
| p | 0 | 0 | 0 |
Note: *p<0.05, #p<0.05 and &p<0.05 relative to control group, TFS-L group and TFS-M group, respectively
Table 2: TFS-Mediated Effect on MDA-MB-231 Cell Apoptosis (x̄±s, n=9)
The data from Table 3 displayed miR-1269 expression was increased in MDA-MB-231 cells relative to MCF-10A cells (p<0.05).
| Group | miR-1269 |
|---|---|
| MCF-10A | 1.00±0.13 |
| MDA-MB-231 | 4.24±0.25* |
| t | 73.625 |
| p | 0 |
Note: Compared with MCF-10A cells, *p<0.05
Table 3: miR-1269 Expression In Breast Cancer Cells (x̄±s, n=41)
As shown in Table 4, miR-1269 expression in TFS-L, TFS-M and TFS-H groups was decreased in dose-dependent manner after being compared with control group (p<0.05).
| Group | miR-1269 |
|---|---|
| Control | 1.00±0.00 |
| TFS-L | 0.77±0.06* |
| TFS-M | 0.61±0.05*# |
| TFS-H | 0.44±0.04*#& |
| F | 265.714 |
| p | 0 |
Note: *p<0.05, #p<0.05 and &p<0.05 relative to control group, TFS-L group and TFS-M group, respectively
Table 4: TFS-Mediated effect on miR-1269 Expression in MDA-MB-231 Cells (x̄±s, n=9)
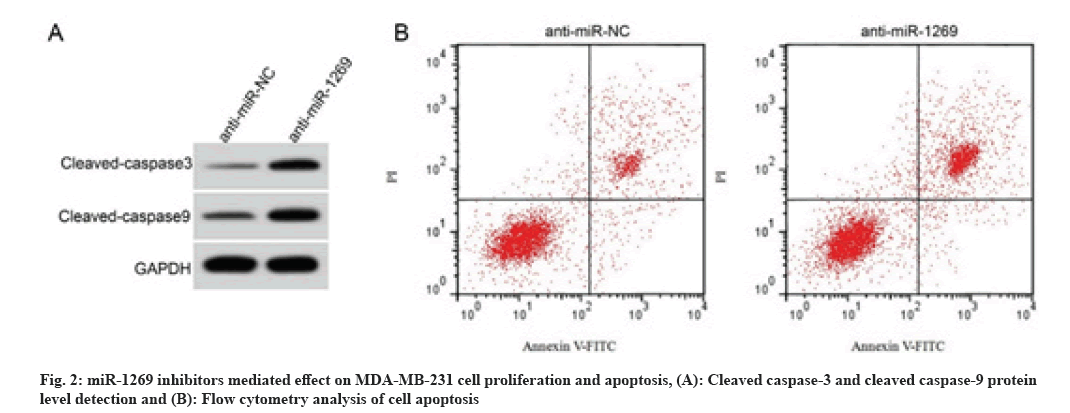
As shown in fig. 2 and Table 5, in comparison with anti-miR-NC group, the cell proliferation inhibition rate, cell apoptotic rates and cleaved caspase-3 and cleaved caspase-9 protein levels were increased in the anti-miR-1269 group (p<0.05), but the number of cell colonies was decreased (p<0.05).
| Group | miR-1269 | Cell proliferation inhibition rate (%) | The number of cell colonies | Apoptotic rates (%) | Cleaved caspase-3 protein expression | Cleaved caspase-9 protein expression |
|---|---|---|---|---|---|---|
| Anti-miR-NC | 1.00±0.00 | 6.27±0.49 | 113.52±12.49 | 6.37±0.56 | 0.15±0.02 | 0.22±0.02 |
| Anti-miR-1269 | 0.33±0.03* | 52.38±5.13* | 63.46±5.13* | 31.95±2.06* | 0.51±0.04* | 0.63±0.05* |
| t | 67.000 | 26.843 | 11.122 | 35.948 | 24.150 | 22.841 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05 relative to anti-miR-NC group
Table 5: miR-1269 Inhibitors Mediated Effect On MDA-MB-231 Cell Proliferation and Apoptosis (x̄±s, n=9)
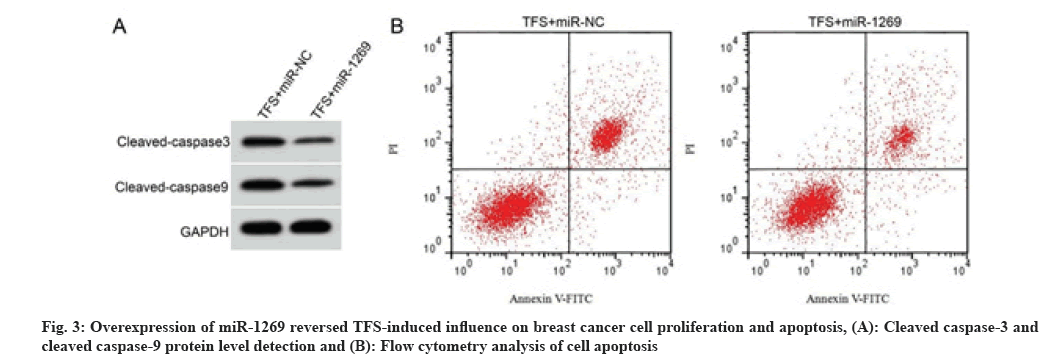
As shown in fig. 3 and Table 6, compared with the TFS+miR-NC group, the cell proliferation inhibition rate, cell apoptosis rate and cleaved caspase-3 and cleaved caspase-9 protein levels were decreased in the TFS+miR-1269 group (p<0.05), whereas the number of cell colonies was increased (p<0.05).
| Group | miR-1269 | Cell proliferation inhibition rate (%) | The number of cell colonies | Apoptotic rate (%) | Cleaved caspase-3 protein expression | Cleaved caspase-9 protein expression |
|---|---|---|---|---|---|---|
| TFS+miR-NC | 1.00±0.00 | 65.13±5.66 | 54.97±4.81 | 29.13±2.01 | 0.58±0.05 | 0.69±0.04 |
| TFS+miR-1269 | 2.66±0.23* | 22.77±2.21* | 94.22±7.16* | 11.09±1.09* | 0.28±0.03* | 0.31±0.03* |
| t | 21.652 | 20.915 | 13.651 | 23.669 | 15.435 | 22.800 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05 relative to TFS+miR-NC group
Table 6: Overexpression of miR-1269 Reversed TFS-Induced Influence on Breast Cancer Cell Proliferation and Apoptosis (x̄±s, n=9)
Traditional Chinese medicine contains a variety of active ingredients and can play an important regulatory role in many diseases. Considerable evidence has revealed that extracts of traditional Chinese medicine can play an anti-breast cancer role by regulating non-coding RNA[11,12]. As endogenous non-coding small RNA molecules, miRNAs are able to modulate the biological behavior of breast cancer cells via binding to target genes and are potential targets for breast cancer treatment[13,14]. However, whether miRNA is a potential target for breast cancer therapy by Chinese herbal extracts has not been clarified.
The extract of S. uncinata (Desv.) spring has anti-tumor effects and its main active component is bioflavonoids, which is able to repress tumor cell growth and metastasis[15,16]. Currently, there is no work on the association between TFS and breast cancer. Our study showed that the increased TFS concentration led to the increased proliferation inhibition rate and the decreased number of colony formation of breast cancer cell lines, suggesting that TFS is able to repress cell proliferation. The activation of the caspase cascade can promote cell apoptosis and the main executive genes of the cascade are caspase-9 and caspase-3, which form cleaved caspase-9 and cleaved caspase-3 respectively after activation and then induce cell apoptosis[17]. Our work revealed TFS promoted the apoptosis rate of breast cancer cells and cleaved caspase-3 and cleaved caspase-9 protein levels in concentration- dependent manner, demonstrating that TFS can promote breast cancer cell apoptosis.
MiR-1269 was up-regulated in gastric cancer tissues and its ectopic expression increased cell proliferation and decreased cell apoptosis[18]. MiR-1269 was up-regulated in liver cancer tissues and cells and its downregulation led to reduced proliferation of liver cancer cells[19]. Our study displayed MDA-MB-231 cells had high miR-1269 expression. MiR-1269 expression was inhibited by TFS stimulation in breast cancer cells in a concentration-dependent manner, suggesting that TFS may play an anti-breast cancer role by inhibiting miR-1269. Also, our results showed that miR-1269 absence could weaken the proliferation and colony formation of breast cancer cell lines and increase cell apoptotic rates, while miR-1269 overexpression could antagonize the effects of TFS on cell proliferation and apoptosis. The above data suggested that TFS can inhibit breast cancer cell tumor properties by down-regulating miR-1269.
In conclusion, TFS can inhibit breast cancer cell proliferation and promote apoptosis and the regulatory of TFS in breast cancer cell phenotypes involved its inhibition in miR-1269 expression. The present results indicate that miR- 1269 has the potential to be a potential target for breast cancer therapy by TFS. Additionally, our results not only lay an experimental foundation in exploring the underlying mechanisms of TFS in breast cancer therapy but also provide a new direction for the research and development of therapeutic drugs for breast cancer. However, it remains to be further explored whether TFS can play an anti-breast cancer role by regulating other non-coding RNAs.
Funding:
The present study was funded by grants from the Fundamental Research Funds for the Jinling Hospital (Grant No: YYQN2021094).
Conflict of interests:
The authors declared no conflict of interests.
References
- Kashyap D, Pal D, Sharma R, Garg VK, Goel N, Koundal D, et al. Global increase in breast cancer incidence: Risk factors and preventive measures. Biomed Res Int 2022;2022:9605439.
[Crossref] [Google Scholar] [PubMed]
- Liu Q, Hodge J, Wang J, Wang Y, Wang L, Singh UP, et al. Emodin reduces breast cancer lung metastasis by suppressing macrophage-induced breast cancer cell epithelial-mesenchymal transition and cancer stem cell formation. Theranostics 2020;10(18):8365.
[Crossref] [Google Scholar] [PubMed]
- Yan W, Ma X, Zhao X, Zhang S. Baicalein induces apoptosis and autophagy of breast cancer cells via inhibiting PI3K/AKT pathway in vivo and in vitro. Drug Des Devel Ther 2018;12(1):3961-72.
[Crossref] [Google Scholar] [PubMed]
- Wu D, Jia H, Zhang Z, Li S. Capsaicin suppresses breast cancer cell viability by regulating the CDK8/PI3K/Akt/Wnt/β-catenin signaling pathway. Mol Med Rep 2020;22(6):4868-76.
[Crossref] [Google Scholar] [PubMed]
- Jiao C, Chen W, Tan X, Liang H, Li J, Yun H, et al. Ganoderma lucidum spore oil induces apoptosis of breast cancer cells in vitro and in vivo by activating caspase-3 and caspase-9. J Ethnopharmacol 2020;247(1):112256.
[Crossref] [Google Scholar] [PubMed]
- Lu Y, Ding Y, Wei J, He S, Liu X, Pan H, et al. Anticancer effects of traditional Chinese medicine on epithelial-mesenchymal transition (EMT) in breast cancer: Cellular and molecular targets. Eur J Pharmacol 2021;907:174275.
[Crossref] [Google Scholar] [PubMed]
- Sheng L,Lin H,Qiu N. Study on total flavonoids from Selaginella uncinata in inhibiting malignant behavior of colorectal cancer cells through circular RNA circ_0006528 pathway. Int J Clin Pract 2022;26(4):106-13.
- Shu H, Mao Z, Yang Y. Inhibition effect of total flavones of Cuiyuncao on lung cancer cells. Pharm Clin Chin Mater Med 2019;10(1):27-9.
- Bai X, Wang Q, Rui X, Li X, Wang X. Upregulation of miR-1269 contributes to the progression of esophageal squamous cell cancer cells and is associated with poor prognosis. Technol Cancer Res Treat 2021;20:1533033820985858.
- Zhang JH, Yu JH. Effect of total flavones of Selaginella uncinata (Desv.) spring on proliferation, apoptosis and glycolysis in gastric cancer cells. World Chin J Digestol 2020;28(22):1121-7.
- Jiao L, Wang S, Zheng Y, Wang N, Yang B, Wang D, et al. Betulinic acid suppresses breast cancer aerobic glycolysis via caveolin-1/NF-κB/c-Myc pathway. Biochem Pharmacol 2019;161:149-62.
[Crossref] [Google Scholar] [PubMed]
- Zhang J, Yu K, Han X, Zhen L, Liu M, Zhang X, et al. Paeoniflorin influences breast cancer cell proliferation and invasion via inhibition of the Notch-1 signaling pathway. Mol Med Rep 2018;17(1):1321-5.
[Crossref] [Google Scholar] [PubMed]
- Yin Z, Wang W, Qu G, Wang L, Wang X, Pan Q. MiRNA-96-5p impacts the progression of breast cancer through targeting FOXO3. Thorac Cancer 2020;11(4):956-63.
[Crossref] [Google Scholar] [PubMed]
- Hua B, Li Y, Yang X, Niu X, Zhao Y, Zhu X. MicroRNA-361-3p promotes human breast cancer cell viability by inhibiting the E2F1/P73 signalling pathway. Biomed Pharmacother 2020;125(2):109994-110004.
[Crossref] [Google Scholar] [PubMed]
- Sun Y, Chen K, Liu Z. Inhibiting action of total flavones from Selaginella uncinata on COX-2 mRNA expression in HT-29 cells. Chin Pharm J 2010;13(2):163-4.
- Su H, Sun H, Li L. Inhibition effect of total alkaloid of Selaginella uncinata (Desv.) spring on S180 solid tumor in mice. Guangxi Sci Technol 2015;22(6):646-50.
- Han J, Pu Y, Liu H. Effects of SKA1 on cell proliferation and apoptosis of renal cell carcinoma and its molecular mechanism. Pract Oncol J 2020;34(5):404-11.
- Liu WL, Wang HX, Shi CX, Shi FY, Zhao LY, Zhao W, et al. MicroRNA-1269 promotes cell proliferation via the AKT signaling pathway by targeting RASSF9 in human gastric cancer. Cancer Cell Int 2019;19(1):308-18.
[Crossref] [Google Scholar] [PubMed]
- Yang XW, Shen GZ, Cao LQ, Jiang XF, Peng HP, Shen G, et al. MicroRNA-1269 promotes proliferation in human hepatocellular carcinoma via downregulation of FOXO1. BMC Cancer 2014;14(1):909-19.
[Crossref] [Google Scholar] [PubMed]