- *Corresponding Author:
- H. Padh
Sardar Patel University, Vallabh Vidhyanagar-388 120, India
E-mail: hpadh@yahoo.com
| Date of Submission | 23 November 2016 |
| Date of Revision | 27 February 2017 |
| Date of Acceptance | 16 June 2017 |
| Indian J Pharm Sci 2017;79(4):655-662 |
Abstract
The extensive demand of large scale therapeutic protein production in Chinese Hamster Ovarian cell lines increased the need of developing efficient and cost effective transfection reagent. Polyethylenimine has recently emerged as an attractive alternative to currently employed conventional transfection methods. In the present report, we attempted to develop inexpensive and easy to prepare polyethylenimine-based transfection reagent and compared its transfection efficiency with calcium phosphate, electroporation and lipoplex mediated transfection methods. Our results indicated highest transfection efficiency with in-house prepared polyplexes as marked by maximum percentage of enhanced cyan fluorescence protein positive cells. These findings contribute towards the development of economical and effective solution for high protein expression in Chinese hamster ovarian system.
Keywords
CHO-K1 cells, delivery efficiency, DNA, ECFP, PEI, polyplex, transfection
Recombinant therapeutic protein production has revolutionized the modern medical sciences. The ever expanding need of therapeutic proteins, demands large scale production and expression of proteins in their active form [1]. Optimum transfection of DNA encoding recombinant protein in an appropriate “host” is critical to meet aforesaid requirements. Chinese hamster ovarian (CHO) cells have been the preferred choice over other existing mammalian expression systems [2]. CHO-K1 cells offer myriad advantages such as potential to express complex bio therapeutics, easy maintenance and adaptation in suspension culture. Moreover, CHO-K1 cell line is resistant to human pathogens and considered to be safe for production of therapeutic proteins with minimum regulatory concerns [3]. Aiming towards faster and inexpensive production of proteins in CHO-K1 system, transient gene expression (TGE) has demonstrated its advantages over the standard stable cell line development process. However, low efficiency in transfection of cells limits its utility in large scale production of therapeutic proteins [4]. Furthermore, it requires large quantity of consumables including transfection reagents and plasmid DNA for every batch, which increase the final production cost of therapeutic proteins [5].
For TGE using CHO cells, viral and non-viral transfection methods have been employed. In comparison to viral vectors, non-viral transfection methods such as electroporation, calcium phosphate (CaPO4) co-precipitation, lipoplex and polyplexmediated methods have been benefited in gaining regulatory clearance and thus preferred for therapeutic protein production as well as for gene therapy [6]. However, these non-viral methods vary with respect to transfection efficiency (Table 1) [7-11] and production cost. Thus, it is important to select an appropriate transfection method, which will be highly efficient and economic.
| Transfection Method |
%Transfection efficiency |
References |
|---|---|---|
| Electroporation | 35-45a | Liu et al.[7] |
| PEI | 20-50b | Hsu et al.[8]; Ehrhardt et al.[9]; Liu et al.[7] |
| TransIT® | 50-60c | [10] |
| CaPO4 | 8-60d | Jordan et al.[11] |
a,b,cPercentage transfection efficiency calculated based on % EGFP positive cells; dpercentage transfection efficiency was determined by staining β-galactosidase expressing cells with X-Gal
Table 1: Overview of transfection efficiency of different transfection methods
With the aim to develop cost effective and efficient alternative, present study reports the formulation of polyethylenimine (PEI) based transfection reagent and assess its stability. To the best of our knowledge, for the first time, we have compared major non-viral transfection methods i.e. CaPO4, electroporation, lipoplex reagent (TransIT®, Mirus Bio. LLC) with in-house prepared polyplex reagent for optimal transfection efficiency in context to CHO cells. A number of biocompatible cationic polymers are available to prepare polyplex, such as PEI [12], polysaccharides [13], and poly(β-amino esters) [14]. Since, PEI is comparatively more stable and efficient in transfection [15], it was selected for preparation of in-house polyplexes. To compare the transfection efficiency of aforesaid methods using CHO-K1 cells, plasmid pECFP-N1 harbouring enhanced cyan fluorescent protein (ECFP) gene was used for transfection.
Polyplexes were prepared by mixing pECFP-N1 and PEI (Branched, MW 25 kDa) at different N/P ratio as described by Boussif et al. [16]. Briefly, the N/P ratios were calculated based on PEI nitrogen per nucleic acid phosphate (1 μg of DNA is 3 nM of phosphate and 1 μl of PEI stock solution contains 10 nM of amine nitrogen). Appropriate amount of plasmid DNA and polymer solution were mixed and vortexed. The resulting polyplexes were incubated for 1 h at room temperature before use.
Particle size of different polyplexes was measured using Zetasizer Nano ZS90 (Malvern Instrument, UK). In order to determine particle size, polyplexes were diluted to 1 ml with 0.9% saline. Stability of the formulated polyplexes (pECFP-N1 and PEI) preparations were assessed by ultrasonication and DNase I treatment. Further, to evaluate the stability of polyplexes in Dulbecco’s Modified Eagle’s medium (DMEM) containing serum, polyplexes were incubated in complete medium up to 6 d.
Prepared polyplexes were subjected to probe ultrasonication on ice bed with relaxation time interval of 20 s after every cycle of ultrasonication (15 s) with amplitude of 2 μ. Samples were collected at time intervals of 15 s, 30 s and 60 s. Finally, to substantiate the integrity of plasmid pECFP-N1, samples were analysed by loading on 0.8% agarose gel stained with ethidium bromide in 0.5×Trisacetate- ethylenediaminetetraacetic acid (TAE) buffer and electrophoresed at 80 V for 40 m and examined under ultraviolet (UV) irradiation. Further, to ascertain presence of intact plasmid DNA in the prepared complex, polyplexes were treated with 4 IU of heparin. After heparin treatment, polyplexes were analysed on agarose gel electrophoresis as mentioned above. To determine whether prepared polyplex can protect the pECFP-N1 from nuclease digestion, polyplexes were incubated with DNase I in DNase I buffer (pH 7.5) at 37° for different time intervals (0 to 180 m). The reaction was terminated at different time intervals by addition of 0.5 M ethylenediaminetetraacetic acid (EDTA). To ensure the presence of pECFP-N1, polyplexes were treated with 4 I.U. of heparin. Samples were then subjected to agarose gel electrophoresis and visualize by UV irradiation as described above. Prepared polyplexes were incubated in DMEM medium containing 10% fetal bovine serum (FBS) at 37° and 5% CO2 up to 6 d. After incubation, polyplexes were dissociated with heparin treatment and were analysed by agarose gel electrophoresis. The prepared polyplexes were evaluated for cytotoxicity by 3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. CHO-K1 cell line was procured from National Centre For Cell Science (NCCS), Pune, India and maintained in DMEM medium containing 10% FBS at 37° in 5% CO2. CHO-K1 cells were seeded at the density of 1×106 cells in 24 well plates and incubated for 24 h. Polyplexes were then added to CHO-K1 cells and incubated for 48 h. After 48 h, 100 μl of freshly prepared MTT (5 mg/ml) solution was added and incubated for 4 h at 37°. MTT was removed and 2 ml of dimethyl sulfoxide (DMSO) was added to dissolve the formazan crystals, which were spectrophotometrically measured at 570 nm. The cytotoxicity experiment was performed in triplicate.
To evaluate the efficiency of different transfection methods, CHO-K1 cells were transfected with pECFP-N1 using CaPO4, liposomes (TransIT®), electroporation and in-house polyplex preparation. CHO-K1 cells were seeded at a density of 3×105 cells per well in 6 well cell culture plate before 24 h of transfection. CaPO4-DNA co-precipitate was prepared as described by Jordan et al. [12] and added to CHO-K1 cells. The cells were then incubated at 37° in 5% CO2.
After 3 h, the medium in each well was replaced with 1 ml of 10% glycerol in phosphate buffer saline (PBS). The cells were then washed with PBS and 2 ml of fresh DMEM with 5% FBS was added.
For electroporation, 5 μg of pECFP-N1 was added to 400 μl of cell suspension (2×106 cells/ml) in a 2 mm cuvette. Mixture of CHO-K1 cells and plasmid DNA were electroporated with Gene Pulser Xcell (Bio-Rad, USA). The parameters for electroporation were set as follows; 580 V, 50 Ω and 50 μF. Transfected cells were seeded on a 24 well plate after electroporation. The electroporated cells were incubated at 37° and 5% CO2. Cells were seeded at a density of 5×105 cells per well in 6 well cell culture plate for 24 h. After incubation, polyplexes were added to each well and incubated for 48 h. Naked pECFP-N1 and PEI were included as controls in the experiment.
A day prior to transfection, 2×105 cells/well were cultured in 6 well plate containing DMEM and 10% FBS. Transfection protocol was performed as described by manufacturer. Briefly, 2.5 μg of pECFP-N1 was added to 250 μl of serum free media supplemented with 7.5 μl TransIT®-CHO reagent and 1.25 μl CHO Mojo reagents. This mixture was incubated at room temperature for 15-30 min, which was added to previously plated cells and further incubated for 24-72 h. To evaluate the best transfection method for the delivery of ECFP gene inside the CHO-K1 cells, fluorescence-activated cell sorting (FACS) analysis (FACS, Becton Dickson, France) was performed after 72 h of transfection.
Statistical analysis was performed using Graph Pad prism (trial version 5). One way ANOVA with Bonferroni’s multiple comparison test was used to determine the statistical significance among the experimental groups. Cytotoxicity data are presented as mean±standard error of mean (SEM) whereas FACS analysis data are presented as mean±standard error (SE). P values of <0.05 were considered as significant for all the statistical analyses.
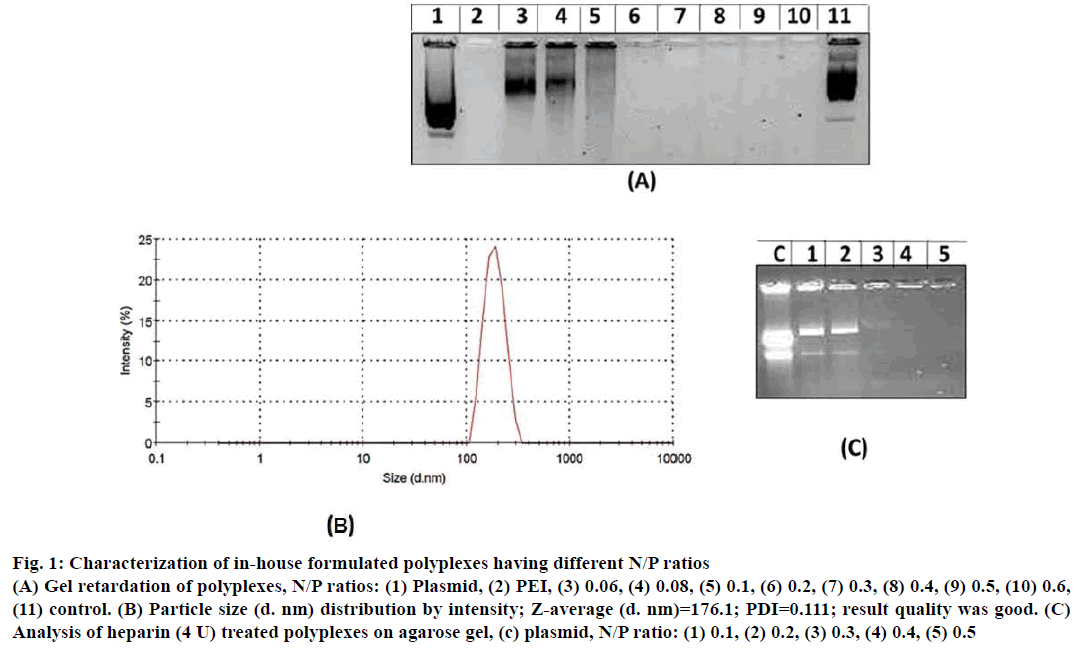
In gel retardation assay, prepared polyplexes of different N/P ratios when electrophoresed, polyplexes having 0.2, 0.3, 0.4, 0.5 and 0.6 N/P ratio were retained in the agarose wells (Figure 1A). Whereas, polyplexes with <0.2 N/P ratios migrated freely towards positive electrode. Particle size and size distribution of polyplexes having different N/P ratios were analysed using Zetasizer (Figure 1B). Polyplexes having <0.2 N/P ratios are smaller in size (80 nm to 145 nm) however; they were unable to condense pECFP-N1. Polyplexes having N/P ratio >0.2 were of 175 nm to 290 nm size and could successfully condense pECFP-N1 as revealed by gel retardation assay. Condensation of pECFP-N1 in formulated polyplexes was confirmed by release of plasmid DNA after heparin (4 IU) treatment as depicted in Figure 1C. As evident in Figure 2A, lane-2, ultrasonication of polyplexes for 60 s did not result in degradation of plasmid DNA, while ultrasonication burst of 15 s resulted in fragmentation of naked plasmid DNA (lane-1, Figure 2A).
Figure 1: Characterization of in-house formulated polyplexes having different N/P ratios
(A) Gel retardation of polyplexes, N/P ratios: (1) Plasmid, (2) PEI, (3) 0.06, (4) 0.08, (5) 0.1, (6) 0.2, (7) 0.3, (8) 0.4, (9) 0.5, (10) 0.6,
(11) control. (B) Particle size (d. nm) distribution by intensity; Z-average (d. nm)=176.1; PDI=0.111; result quality was good. (C)
Analysis of heparin (4 U) treated polyplexes on agarose gel, (c) plasmid, N/P ratio: (1) 0.1, (2) 0.2, (3) 0.3, (4) 0.4, (5) 0.5
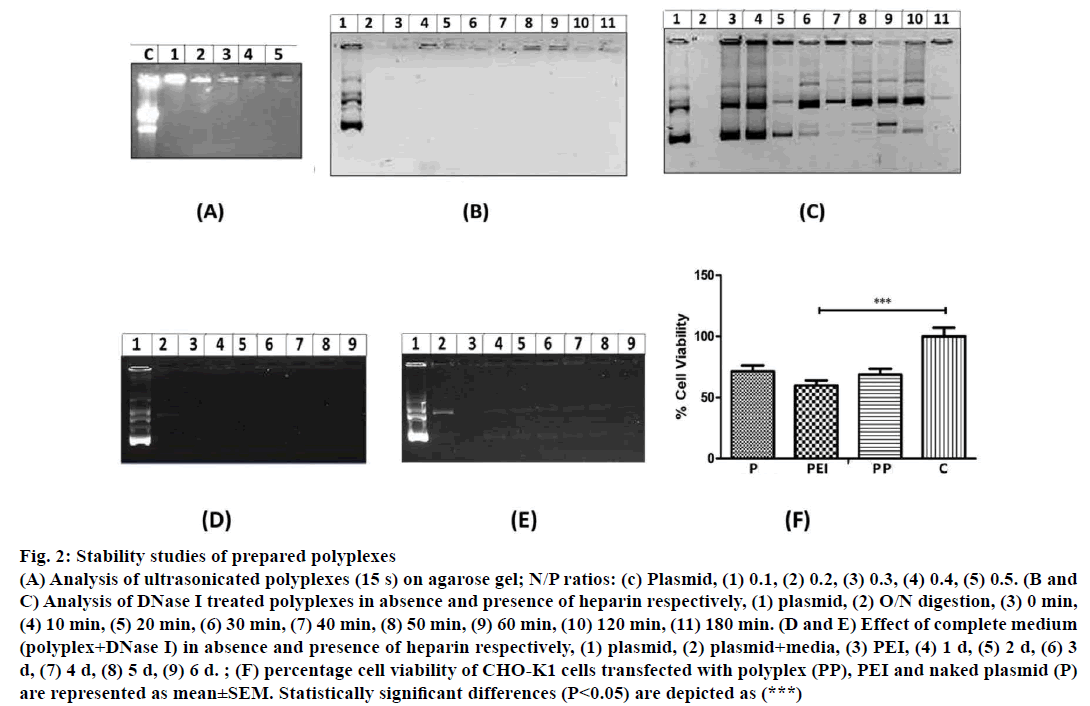
Figure 2: Stability studies of prepared polyplexes
(A) Analysis of ultrasonicated polyplexes (15 s) on agarose gel; N/P ratios: (c) Plasmid, (1) 0.1, (2) 0.2, (3) 0.3, (4) 0.4, (5) 0.5. (B and
C) Analysis of DNase I treated polyplexes in absence and presence of heparin respectively, (1) plasmid, (2) O/N digestion, (3) 0 min,
(4) 10 min, (5) 20 min, (6) 30 min, (7) 40 min, (8) 50 min, (9) 60 min, (10) 120 min, (11) 180 min. (D and E) Effect of complete medium
(polyplex+DNase I) in absence and presence of heparin respectively, (1) plasmid, (2) plasmid+media, (3) PEI, (4) 1 d, (5) 2 d, (6) 3
d, (7) 4 d, (8) 5 d, (9) 6 d. ; (F) percentage cell viability of CHO-K1 cells transfected with polyplex (PP), PEI and naked plasmid (P)
are represented as mean±SEM. Statistically significant differences (P<0.05) are depicted as (***)
DNase I treatment of polyplexes at various time intervals (0 to 180 min) did not exhibit degradation of pECFP-N1 (Figure 2B) whereas naked plasmid DNA was degraded upon DNase I treatment. Similarly, plasmid DNA was also found to be degraded when heparin treated polyplexes were subjected to DNase I (lane 5 to 11, Figure 2C). These observations indicate that the formulated polyplexes can provide adequate protection to pECFP-N1 from DNase I and thus ensuring in vitro and in vivo protection of plasmid from nucleases. Culture medium used for transfection of mammalian cells contains serum having various growth factors and hormones, which may affect the stability of polyplexes. Hence, to analyse the integrity of plasmid, prepared polyplexes were incubated with DMEM medium containing 10% FBS and DNase I up to 6 d and analysed by agarose gel electrophoresis. As revealed in Figure 2D, the formulated polyplexes were found to be stable even at the end of 6 d incubation. The release of plasmid and degradation from polyplexes was observed only after the treatment with heparin (Figure 2E). Polyplexes were subjected to MTT assay for evaluation of cytotoxicity. As evident from Figure 2F, around 68% cell viability was observed with formulated polyplexes indicating the non-toxic performance of formulation.
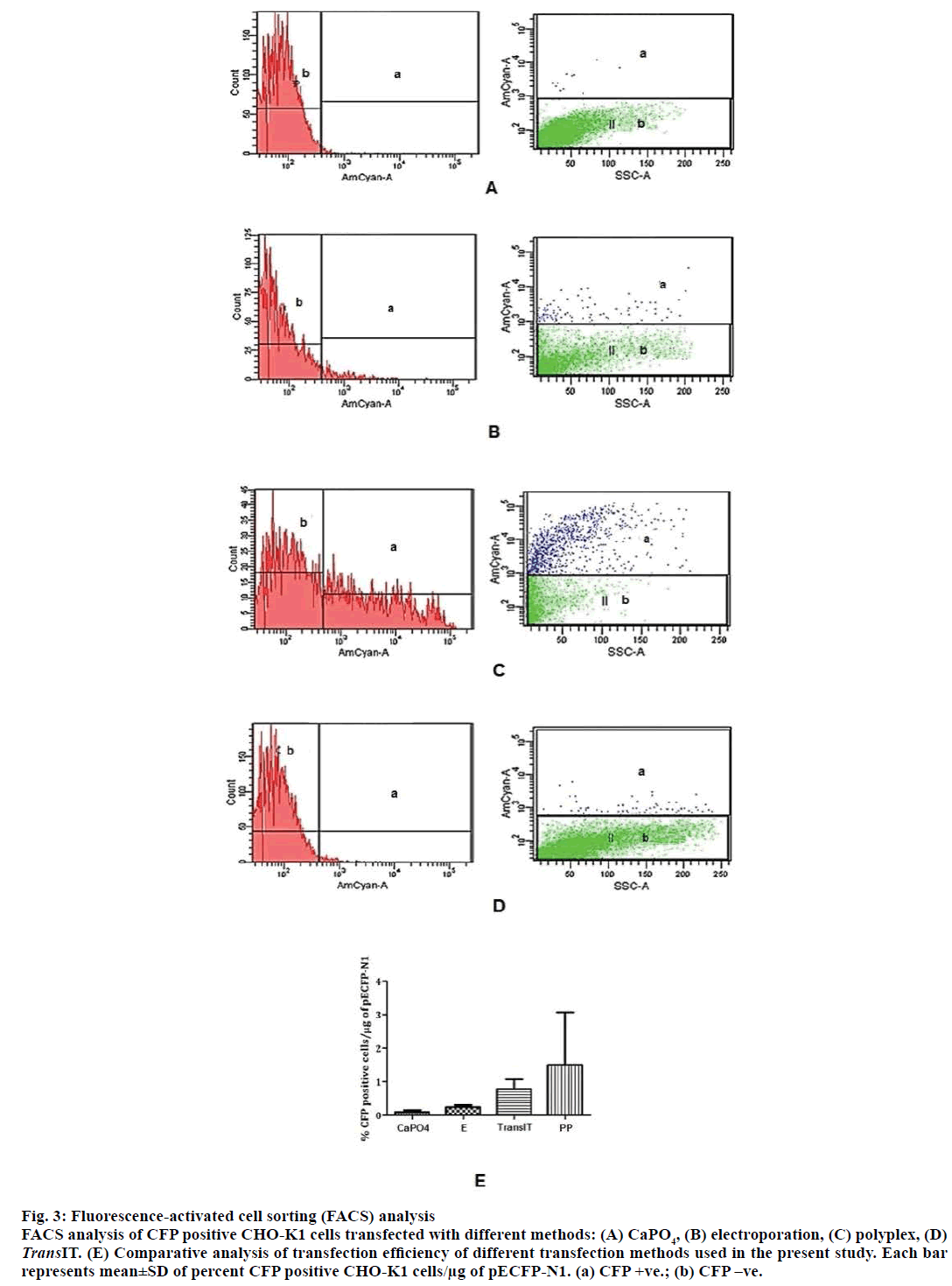
To compare the transfection efficiency of inhouse prepared polyplexes with other conventional transfection methods viz. CaPO4, commercially available lipoplex reagent and electroporation, CHO-K1 cells were transfected with pECFP-N1. ECFP expression was monitored in transfected CHO-K1 cells. The transfection efficiencies of different methods were calculated as percentage ECFP expressing CHO-K1 cells per 1 μg of DNA. The highest percentage of ECFP positive cells were observed in case of transfection with polyplexes (Figure 3). The comprehensive summary of the entire study is depicted in Figure 4.
Figure 3: Fluorescence-activated cell sorting (FACS) analysis
FACS analysis of CFP positive CHO-K1 cells transfected with different methods: (A) CaPO4, (B) electroporation, (C) polyplex, (D) TransIT. (E) Comparative analysis of transfection efficiency of different transfection methods used in the present study. Each bar
represents mean±SD of percent CFP positive CHO-K1 cells/μg of pECFP-N1. (a) CFP +ve.; (b) CFP –ve.
For efficient transfection of mammalian cells, numerous transfection methods have been devised. Among which electroporation, CaPO4 and lipoplex mediated transfection methods are routinely practiced. However, their mode of DNA delivery, efficiency and experimental cost vary to a considerable extent [4]. Even though, transfection with aforesaid methods results in gene expression, either high experimental cost or low transfection efficiency poses major obstacle for large scale transfection [17]. In the search of cost effective and efficient alternative, we have formulated in-house polyplexes of PEI as a transfection reagent and compared its transfection efficiency with other conventional methods such as electroporation, CaPO4 and lipoplex mediated transfection method.
PEI, an organic polymer, can spontaneously adhere to DNA and condense it into toroidal complexes, which can be easily endocytosed by cells. The condensation of DNA is dependent on the electrostatic interactions between PEI and DNA. With the increase in molecular weight of PEI, the condensation ability and surface charge increase and complex size decreases [18]. Considering these facts, we have exploited one of the highest molecular weight PEI (25 kDa), which successfully condensed pECFP-N1 as confirmed by gel retardation assay. The present study also evaluated the stability of PEI-pECFP-N1 complex and gene delivery efficiency of formulated complex, which are important criteria for the use of PEI as a transfection reagent. The PEI-DNA complex was stable enough to prevent DNA dissociation even after 60 s probe sonication treatment indicating the stability of formulated polyplexes.
Gene delivery in mammalian cells is often susceptible to nucleases, which results in low transfection efficiency [19]. To circumvent the DNA degradation, PEI is known to play an important role, which has been strengthen by our findings [18]. The toroidal complex of PEI-pECFP-N1 could successfully protect pECFP-N1 from degrading action of DNase I enzyme up to 6 d whereas only transient incubation of DNase I with naked DNA resulted in complete degradation. This indicates protective nature of formulated polyplexes with respect to DNase I activity, reinforcing the suitability for in vitro and in vivo transfection. Transfection of mammalian cells with plasmid DNA is generally carried out in a cultivation medium supplemented with serum, which is required for cell proliferation and maintenance [20]. Serum contains several growth factors, which might destabilize the prepared polyplexes leading to reduction in transfection efficiency, thus we have evaluated the stability of prepared polyplexes in a complete medium at various time intervals. It was found stable PEIplasmid complex even after 6 d of incubation. This characteristic of formulated polyplexes renders its prospective application as a transfection reagent.
The use of polymer as a transfection reagent may result in cytotoxicity [21]. Hence, it is prudent to evaluate the cytotoxicity of in-house prepared polyplexes before its application as a transfection reagent. The observed cytotoxicity of prepared polyplexes was comparable to that of naked DNA indicating its suitability in mammalian cell transfection. The transfection efficiency attained by different transfection methods may vary greatly [22]. Upon comparison of transfection efficiency of methods employed in present study, the in-house formulated polyplexes was found to be highly efficient as revealed by maximum percentage of ECFP positive cells. Various factors contributing towards the greater efficiency of PEI mediated transfection in CHO-K1 cells could be; stability of polyplexes in serum, small sized polyplexes for efficient endocytosis by host cell, protection from DNase I and less toxic to cultured cells [15,16]. High transfection efficiency with PEI based method has also been reported to be 40-90% by other researchers [15].
Though, CaPO4 is a well-established and inexpensive method, it does not work efficiently with CHO cells as evident by our results, which are in line with the previous findings [23]. Lipoplex mediated transfection demonstrates high transfection efficiency in delivering plasmid DNA into wide range of mammalian cells [24]. On the contrary, our results showed greater transfection efficiency with polyplexes as compared to lipoplex (TransIT®), which could be because of differences in the composition of lipoplex and polyplex used. Moreover, high cost discourages the routine usage of lipoplex in large scale protein production. Similarly, electroporation method is also unsuitable for large scale cell transfection [22].
These observations of previous reports strengthen the findings of the present studies. Production of heterologous proteins in CHO cells is in high demand and gaining global attention [17]. Higher expression of therapeutic proteins could meet the increasing requirement, which could be achieved through efficient transfection reagents. In this regard, we have successfully designed polyplexes, which resulted in high transfection efficiency. Our polyplexes can be prepared in few steps using inexpensive reagents and can be stored for longer time. Further, evaluation of polyplexes for transfection of other cell lines could diversify the prospective application of formulated polyplexes. The promising results with different cell lines would encourage the commercialization of PEI based transfection reagent.
Acknowledgement
We would like to acknowledge B. V. Patel PERD Centre and CPL Biologicals for providing the infrastructure and resources. Bhrugu Yagnik is a recipient of Lady Tata Memorial Trust’s Fellowship.
Financial assistance
None.
Conflict of interests
None declared.
References
- Desai PN, Shrivastava N, Padh H. Production of heterologous proteins in plants: strategies for optimal expression. Biotechnol Adv 2010;28:427-35.
- Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol 2004;22:1393-8.
- Jayapal K, Wlaschin K, Hu W, Yap G. Recombinant protein therapeutics from CHO cells-20 years and counting. Chem Eng Prog 2007;103:40-7.
- Zhu J. Mammalian cell protein expression for biopharmaceutical production. Biotechnol Adv 2012;30:1158-70.
- Cheng L, Sun X, Yi X, Zhang Y. Large-scale plasmid preparation for transient gene expression. Biotechnol Lett 2011;33:1559-64.
- Nayerossadat N, Ali P, Maedeh T. Viral and nonviral delivery systems for gene delivery. Adv Biomed Res 2012;1:27.
- Liu YC, Lin WY, Jhang YR, Huang SH, Wu CP, Wu HT. Efficiency of DNA transfection of rat heart myoblast cells H9c2 (2-1) by either polyethyleneimine or electroporation. Appl Biochem Biotechnol 2011;164:1172-82.
- Hsu CY, Uludağ H. A simple and rapid nonviral approach to efficiently transfect primary tissue-derived cells using polyethylenimine. Nat Protoc 2012;7:935-45.
- Ehrhardt C, Schmolke M, Matzke A, Knoblauch A, Will C, Wixler V, et al. Polyethylenimine, a cost‐effective transfection reagent. Signal Transduct 2006;6:179-84.
- https://www.mirusbio.com/assets/product-data-sheets/transduceit-reagent-pds.pdf.
- Jordan M, Schallhorn A, Wurm FM. Transfecting mammalian cells: optimization of critical parameters affecting calcium-phosphate precipitate formation. Nucleic Acids Res 1996;24:596-601.
- Jordan M, Schallhorn A, Wurm FM. Transfecting mammalian cells: Optimization of critical parameters affecting calcium-phosphate precipitate formation. Nucleic Acids Res 1996;24:596-601.
- Mao HQ, Roy K, Troung-Le VL, Janes KA, Lin KY, Wang Y, et al. Chitosan-DNA nanoparticles as gene carriers: Synthesis, characterization and transfection efficiency. J Control Release 2001;70:399-421.
- Jere D, Kim TH, Arote RB, Jiang HL, Cho MH, Nah JW, et al. A poly(β-amino ester) of spermine and poly(ethylene glycol) diacrylate as a gene carrier. Key Eng Mater 2007;342-3:425-8.
- Ehrhardt C, Schmolke M, Matzke A, Knoblauch A, Will C, Wixler V, et al. Polyethylenimine, a cost-effective transfection reagent. Signal Transduct 2006;6:179-84.
- Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA 1995;92:7297-301.
- Hacker DL, De Jesus M, Wurm FM. 25 years of recombinant proteins from reactor-grown cells - Where do we go from here? Biotechnol Adv 2009;27:1023-7.
- Mady MM, Mohammed WA, El-Guendy NM, Elsayed AA. Effect of polymer molecular weight on the DNA/PEI polyplexes properties. Rom J Biophys 2011;21:151-65.
- Arangoa MA, Du N. Enhanced gene delivery in vitro and in vivo by improved transferrin-lipoplexes. Biochim Biophys Acta 2002;1561:209-21.
- Moret I, Esteban Peris J, Guillem VM, Benet M, Revert F, Dasí F, et al. Stability of PEI-DNA and DOTAP-DNA complexes: Effect of alkaline pH, heparin and serum. J Control Release 2001;76:169-81.
- Brunot C, Ponsonnet L, Lagneau C, Farge P, Picart C, Grosgogeat B. Cytotoxicity of polyethyleneimine (PEI), precursor base layer of polyelectrolyte multilayer films. Biomaterials 2007;28:632-40.
- Kim TK, Eberwine JH. Mammalian cell transfection: The present and the future. Anal Bioanal Chem 2010;397:3173-8.
- Batard P, Jordan M, Wurm F. Transfer of high copy number plasmid into mammalian cells by calcium phosphate transfection. Gene 2001;270:61-8.
- Ross PC, Hui SW. Lipoplex size is a major determinant of in vitro lipofection efficiency. Gene Ther 1999;6:651-9.