- *Corresponding Author:
- N. K. Jain
Pharmaceutics Research Laboratory, Department of Pharmaceutical Sciences, Dr. Hari Singh Gour University, Sagar - 470 003, India.
E-mail: jnarendr@yahoo.co.in
| Date of Submission | 8 March 2006 |
| Date of Revision | 14 March 2007 |
| Date of Acceptance | 2 July 2007 |
| Indian J Pharm Sci 2007, 69 (4): 479-488 |
Abstract
Ultra thin multilayer capsules are attractive and stable systems capable of delivering the bioactives. Ultra thin multilayer capsules consist of polyelectrolytic materials that are formed in the presence of a template. This is achieved through layer-by-layer adsorption of oppositely charged macromolecules on to colloidal particles. Upon extraction, the resulting cavities retain affinity for the bioactives. This review considers the fabrication, of ultra thin multilayer capsules, physiochemical properties and role of ultra thin multilayer capsules within a pharmaceutical remit. Ultrathin multilayer capsules have potential for creating satisfactory drug dosage forms.
tipobet vdcasino venüsbet sahabet sekabet sahabet onwin matadorbet casibom casibom casibom casinoplus casibom casibom jojobet jojobet jojobet grandpashabet grandpashabet grandpashabet
Keywords
Ultra thin multilayer capsules, drug delivery, polyelectrolyte, dendrimer.
Supramolecular science is the branch, which has become the most fast developing field especially with development of peptide synthesis and supramolecular self-assemblages as drug delivery system. During last few years, supramolecular chemistry has resulted into the development of many dynamic supramolecular systems [1].
A novel approach in this regard is the development of smart, functional and organized supramolecular materials like various types of hosts, which will find ways to the specific receptor; and self-assembling nanostructures to be used as drug delivery system. Decher and Hong have proposed alternative adsorption of polyanion and polycation about a decade ago [2,3]. The principle of multilayer assembly is as follows: a solid charged substrate is immersed in a solution of oppositely charged polyelectrolyte solution and a layer of poly ion is adsorbed via electrostatic attraction. Since adsorption is carried out at relatively high concentration of polyelectrolyte, a number of oppositely charged groups remain exposed to the solution and thus repeating this procedure effectively reverses surface charge and alternating multilayer assembly is obtained [4].
The idea of applying this procedure for production of films with alternation of two or more poly ion components was first realized for linear polymer ions and spatial separation of neighboring poly ion layers with minor interpenetrating was confirmed from neutron reflexivity experiments [5]. Electrostatic alternate adsorption was successfully employed for low molecular weight dyes and assembling process of individual dye-poly ion layer of Congo red-Poly (diallymethyl ammonium chloride) was investigated by using a quartz crystals microbalance [6].
Multicomposite ultra thin capsules are molecular assemblies of tailored architecture having layer-bylayer adsorption of oppositely charged macromolecules on to colloidal particles [7]. At present, a variety of materials, such as wide range of synthetic polyelectrolytes, biopolymers, lipids and inorganic particles have successfully been employed to fabricate multilayered films on the flat substrate by taking advantage of electrostatic interaction between oppositely charged species in their stepwise adsorption from an aqueous solution [3,4,8].
Polyelectrolyte shells with controlled thickness and composition were fabricated by stepwise adsorption of oppositely charged polyelectrolytes on to melamine resin templates, which are subsequently solubilized. Recently, this technology has also applied for multilayer assembling on the surface of colloidal particles, subsequently removal of core leads to the formation of very stable hollow structures [9,10]. Such hollow thin walled microcapsules have attracted particular interest from viewpoint of application in encapsulation (for example as drug carrier system or micro-reactors).
In 2001, Qiu et al. reported that encapsulation of ibuprofen drug crystals by a novel coating technology based on layer by layer assembly of oppositely charged polyelectrolytes using biocompatible polyelectrolytes like chitosan, dextran sulphate, carboxymethylcellulose and sodium alginate, substantially prolonged the release of the encapsulated drug [11]. Khopade and Caruso prepared electrostatically assembled polyelectrolyte/dendrimer multilayer films in to ultra thin nanoreservoirs [12]. Bhadra et al. prepared multicomposite ultra thin capsules for sustained ocular delivery of ciprofloxacin hydrochloride and concluded that the capsules can be considered suitable and safe for ocular drug delivery [7].
Fabrication of Umcs
While understanding structural and chemical interaction between host-guest systems, self-assembling system is essential for designing molecules that can mimic natural substrate. At present, a variety of materials, such as wide range of synthetic polyelectrolytes, biopolymers, lipids and inorganic particles have successfully been employed to fabricate multilayered films on the flat substrate or surface of colloidal particle by taking advantage of electrostatic interaction between oppositely charged species in their stepwise adsorption from an aqueous solution [2-4,8,13-15].
The most important novel features of the fabricated polyelectrolyte shells are, i. the composition and thickness of the shell can be controlled; ii. they can be fabricated with controlled physical and chemical properties; and iii. they offer novel structure for micro- and nano-compartmentalization of materials.
In addition, unlike liposome structure [9], the fabricated shells are readily permeable by small (Ca 1-2 nm in diameter) polar molecules and are extremely stable against chemical and physical influence. In principle, permeability features of the capsule wall, such as selectivity for water-soluble components, could be used to create a difference with chemical composition between the bulk solution and the capsule interior. This is an intriguing situation concerning possible application of the microcapsules as microreactors or micro-carriers. However, at present little is known about the permeability properties of these free polyelectrolyte films. A convenient approach to study and to employ the selective permeability properties of the capsules wall is to create a Donnan equilibrium situation. For example, if an electrolyte species is excluded from the encapsulation UMCs permeates counter ions, which should be distributed on both sides of shell wall according to Donnan equilibrium. Hence, their concentration should be different on both sides of the capsule. In the case of protons this would correspond to pH difference. Furthermore, the presence of a non-permeating electrolyte species in the solution on one side of capsule wall induces an osmotic pressure difference across the wall. This may eventually lead to volume changes of the hollow polyelectrolyte capsules and can reflect on the mechanical properties of the shell wall [14].
Templates
Different templates with size ranging from 50 nm to few microns, such as organic and inorganic colloidal particles, aggregated proteins, biological cells and drug nanocrystals can be coated with multilayered film. Various materials viz. synthetic polyelectrolyte, chitosan and its derivatives, proteins, DNA, lipids, and magnetic nanoparticles, have been used as layer constituents to fabricate and design the shell to adjust required stability, biocompatibility and affinity between layers of the capsules [16,17]. Some colloidal templates can be decomposed at conditions of where the polymer shell is stable and which leads to the formulation of hollow capsule with defined size, shape and shell thickness.
After the dissolution of core template at low pH, the assembled polyelectrolyte films remain intact [9]. The capsule size and wall thickness are determined by the size of the original colloids and the number of adsorbed polyelectrolyte layers, respectively.
Layer constituents
Polyions, predominantly used in the ultra thin multilayered assembly are shown in Table 1. The use of polyelectrolyte rather than small molecules is advantageous mainly because good adhesion of a layer to the underlying substrate or film requires a certain number of ionic bonds. Therefore, the overcompensation of the surface charge by the incoming layer is more a property of the polymer than a property of the surface. This is because polymer can simply bridge the underlying defect. Their conformation at the surface (and thus also the newly created film surface) is mostly dependent on the chosen polyelectrolytes and adsorption condition and much less dependent on the substrate or the substrate charge density [18,19].
| Polycations | Polyanions |
|---|---|
| Poly (ethyleneimine) (PEI) | Poly (styrene sulfonate) (PSS) |
| Poly (diallydimethyl ammonium) chloride (PDADMAC) | Poly (vinyl sulfate) (PVS) |
| Poly (dimethyldiallyl ammonium chloride) (PDDA) | Poly (acrylic acid) (PAA) |
| Poly (allylamine hydrochloride) (PAH) | Heparin |
| Polylysine | DNA |
| Chitosan | - |
Table 1: different types of polyions used in The ultra thin multilayered assembly
The linear augmentation of film thickness with the number of deposited layers is often similar even if different substrates are used, which makes the film properties rather independent of the substrate. There are cases where substrate charge densities are very small, the first layer binds to the surface with only a few groups and exposes a larger number of oppositely charged groups to the solution. This effective multiplication of surface functionality often continues over a few layers before a linear deposition regime is reached [3].
Similar to this self-regulation of sequential increment of thickness layer, there is a tendency toward a certain value of the interfacial overlap between a polyanionic layer and a polycation and a certain roughness at the film-air interface; these attributes are probably a property of polyanion-polycation pair rather than a property of the substrate. The polyelectrolyte multilayers have similar surface roughness, regardless of the roughness of the underlying substrates. One possible explanation is that the surface roughness of rough polyelectrolytes films can be annealed to smaller values by consecutively immersing films in solutions of salt and pure water [13].
Different methods of UMCs fabrication
A decade ago, Hong and Decher demonstrated the basic principle, i.e. the alternative exposure of a charged substrate to solutions of positive or negative polyelectrolytes, respectively [2]. Provided that each adsorption step leads to charge inversion of the surface, the subsequent deposition finally results in a layered complex, stabilized by strong electrostatic forces. Such self-assembled polyelectrolyte multilayers have proven to be versatile materials with respect to the incorporation of different charged compound or nano-objects [20].
The multilayered microcapsules can be fabricated by three methods such as, centrifugation, sequential adsorption and membrane filtration. All these three methods can now be used to prepare layer-by-layer polyelctrolyte microcapsules (shells) on colloid particles. These methods are comparable with each other. Membrane filtration provides the highest quality of UMCs because it minimizes particle aggregation during the total preparation process of adsorption and washing cycles. The microcapsules are not stressed, with the exception of a permanent aggregation. Its intensity is rather low and has no negative influence on the products. The membrane filtration method has high adaptation capacity to me et al the requirements of a great variety of chemical and colloid chemical systems. The compromise between the quality of the product and the performance as well as yield of the production can be controlled arbitrarily.
The success in the resuspension of the sediment formed in the centrifugation method is strongly dependent on the aggregated strength of the particles. It is rather difficult to resuspend particles of a strong aggregate without calculable modifications, which can damage the prepared surface structure. It is found that in preparing 20 layered UMCs on a PAH/PSS basis, there is an accumulated total loss of about 80% of originally introduced material by centrifugation, whereas comparatively there was less than 3% loss by membrane filtration under appropriate conditions.
The sequential adsorption technique results in a loss of more than half of the original material [10]. In sequential adsorption technique, one has to control the concentration of all the suspension components pedantically during the whole process. Working with excess amounts of adsorbing polyelectrolyte, as can be done successfully with great advantage in centrifugation and filtration, has to be prevented in the sequential adsorption method. Even with exact concentration control, a tendency for aggregation is not avoidable.
In contrast to sequential adsorption, the membrane filtration and centrifugation methods provide excellent results in a wide range of concentration and target particles. In the filtration technique the suspension medium is driven by a hydrodynamic pressure gradient to separate it from the suspension. The centrifugation method exploits separation forces based on the density difference between the particles and the medium. But the filtration method is not sensitive to this density difference between the particle and the medium. Over a wide range it still operates very effectively where the centrifugation fails or is cumbersome.
Controlling the particle interaction is the main problem with all these methods of particle interaction. Usually one has to cope with the tendency of the particles to form more or less strong aggregates during different stages of the preparation cycle.
Mechanism of Umcs Growth
Charge overcompensation and distribution
Several generalizations may be made regarding the formation of layered multi composites by sequential adsorption from polyelectrolyte only, at least for strongly charged polymers of high molecular weight. First, the polyelectrolyte adsorption process is irreversible on the time scale of multilayer assembly, a property that is required for stable multilayer growth [21]. Irreversibility includes the lack of spontaneous desorption, which results from competition with small (salt) ions, and stripping of surface polymer by it’s oppositely charged solution counterpart [22]. Second, the most important variable for determining multilayer thickness (beside the number of layers) is the salt concentration of the solution used for deposition [2,3,18,23,24]. Third, polymer in two-component multilayers is stratified into well-defined layers but is dispersed and interpenetrating [3,5,25].
Additional variables are ranked in rough order, from significant to subtle, as follows: dielectric constant of the deposition solvent, identification of the salt, deposition time, polymer concentration and polymer molecular weight [26]. For weak polyelectrolytes, the degree of ionisation becomes an important consideration [27]. Mechanistically, the importance of surface charge overcompensation and reversal in polyelectrolyte multilayers, UMCs has been emphasized, since each exposure deposits a reproducible quantity of material and reverses the charge on the surface, leaving it primed for the next layer of polymer [2,3,18,23,24,28].
Theoretical treatments of molecule-molecule and molecule-surface charge overcompensation have predicted modest overcompensation [29]. Recent experiments on strongly dissociated polyelectrolyte pairs, such as PDADMA and PSS, have shown that, in the presence of salt, several equivalent monolayers of polyelectrolyte accumulate on the growing multilayers with a single exposure [22].
Extension of classical theory for the adsorption of polymer at impermeable surfaces would not predict a thick layer of polymer [30]. To account for this excess polymer surface, charge distributed over several interpenetrating layers of polycations and polyanions was depicted [22,26].
The role of surface charge in determining and limiting the increment of polymer added per deposition cycle was probed in a recent study using radiolabelled counterions [22]. With the strongly dissociated polyelectrolytes, PSS and PDADMA, a constant surface charge develops during multilayer buildup. The surface charge, propagated and inverted by sequential adsorption steps, is over neutralized (overcompensated) on each step. A steady state of thickness increment Vs layer number appears when the level of overcompensation is constant. In this mechanism, the salt concentration determines adsorbed amount (layer thickness) by controlling surface charge [31].
Internal layering: Implications of intrinsic compensation
In a study it has been found out that small ions do not participate in charge balance with in PSS/ PDADMS multilayers, this feature has been termed as intrinsic compensation [28]. Intrinsic compensation in thick UMCs requires interpenetration of polymers; a significant constraint. For example, it is not possible to have density fluctuations for two components that are intrinsically compensated. For three components, wherein one is labeled, density fluctuations are possible and have been observed, but the material in any nominal layer is actually interdiffused or fuzzy over several layers [3,5]. Well-defined layering would be important for certain applications requiring anisotropic properties, such as conductivity in two dimensions. The following categories of material approaches may lead to distinct layering:
Forced intrinsic compensation
Post-build up processing steps can induce excess polymer charge of one type within the multilayer. Such steps include thermolytic or photolytic elimination of charged groups [32], electrochemical injection of charge into the multilayer [33,34], or the introduction of charge via pH changes when the multilayer includes weakly acidic polyelectrolytes4. It can be observed in poly(phenylenevinylene) containing UMCs [35].
Particle/polymer systems
A mixture of organic polyelectrolyte and inorganic or organic particles would be conducive to stratification due to the rigid, impenetrable nature of the particle. Multilayers incorporating plate like clay minerals can show Bragg diffraction [36], although the minerals may not be localized in one continuous plane.
High ratio neutral/charged systems
As the material balance within a multilayer tends toward a larger fraction of uncharged hydrocarbon (for example, from hydrophobic side chains or repeatedly sparsely charged at units), phase separation is possible. Examples of systems with high neutral/ charge ratio forming layers have been observed [37,38]. Intrinsic compensation is a balance between entropy loss imposed by localizing segments and free energy gained from ion pair formation. One might expect an intrinsic → extrinsic phase transition as fewer ion pairs hold more polymers together.
Minimal thickness layering
If the layer thickness is approximately the dimension of a monomer repeat unit or less, ion pairs and hydrophobic parts can exist within different strata. This mechanism of layering is likely only with rigid polymers.
Factors controlling growth of Umcs
In exploring growth conditions, it is generally observed that salt has the strongest influence on the amount deposited (“layer” thickness) per cycle. Polymer concentration, molecular weight, and deposition time are known to be less important variables [3,18,23]. Various factors controlling the growth conditions on polyelectrolyte deposition were studied including the effect of salt concentration, salt type, exposure time, polymer concentration and solvent quality [26].
Thickness Vs salt concentration
The trends in layers thickness on the rotating substrates were similar to those for static dip-cycled multilayered [2,3,18,23,39]. Layer thickness for the PSS/PDADMA combination, as a function of number of deposition cycles does not depend on whether the multilayers have been dried between layer pairs [40]. For a given number of layers the experimental variable with greatest influence on the amount deposited is the salt concentration. Some authors reported a linear dependence on salt concentration [26,41]. Others found that the thickness of a layer pair of PSS/PAH was proportional to the ionic strength squared40].
Recent work by Losche et al. on the same system has established a linear relationship of layer pair thickness with salt concentration. Significantly, the steady state increment per layer pair for PSS/PDADMA is 7 times higher than the increment for PSS/PAH under same conditions. This demonstrates the importance of polyelectrolyte type in determining ultimate film thickness [41].
Hydrophobicity and solvent quality
The mechanism of interaction is charge compensation and the driving force is the multiplicity of segmentsegment contacts which acts cooperatively [26]. A specific affinity of one polymer segment for another (polymer for surface) is characterized by chemical interaction.
The adsorption of polyelectrolytes to an oppositely charged surface is an ion exchange phenomenon [42], where charged segment replace small (salt) ions compensating the surface charge, as in equation shown below. Pol- and Pol+ are charged polymer segments, Pol- Pol+ is an ion pair, and M+, A- is salt counter ions. Pol- Pol+ (m) + Pol+A- (aq) ⇔ Pol- Pol+ (m) + M+ (aq) + A- (aq), the subscript “m” refers to the multilayer surface or more precisely a region close to the surface.
Since adsorption is considered in terms of ion exchange, it is expected that different ions with differing affinity should modulate the adsorbed amount. In the process of ion exchange, as a rule, the ion that swells the least that the exchanger binds more strongly [43] i.e. the least hydrated ion pair should be most stable. For example, in the alkali metal series Li+→Cs+, the least solvated ion (Cs+) binds the strongest to the sulfonate exchanger. Multivalent species are much stronger than univalent.
The effect of salt type on polymer adsorption can be viewed from two complementary perspectives. First, a stronger binding salt ion would be functionally equivalent to adding more salt. Second, a less solvated and stronger binding ion would decrease the solvation energy of solution polymer, which would drive the polymer to the interface.
An alternative to adding different counter ions to make solvent poorer would be to vary the solvent itself, decreasing the dielectric constant so that the solvation energy for the charged, hydrophilic polyelectrolyte becomes less favorable. This should also drive the polymer to the interface. This shows the effect on multilayer thickness of increasing the organic fraction in a water/ethanol solvent. As ethanol concentration goes beyond 40% the polymers start to precipitate. It is clear that solvent composition offers another degree of control in multilayer formation.
Surface hydrophobicity could be controlled by selection of surface counter ion e.g. aggregation of perfluroalkane chains on the surface, when immersed in solution of perflurooctane sulphonic acid [26].
Kinetics and irreversibility
A radiolabeled positive layer (14C- labeled PM2VP) was adsorbed on the top of a 10-layer pair PSS/ PDADMA multilayer that had been deposited from either 1.0 or 0.1 M NaCl. No evidence for desorption was found on the time scale of hours. After a few days a small fraction of polymer exchanged, and even after several weeks only 30-40% had exchanged. The desorption/exchange results were similar for both salt concentrations [26].
In a study by Dubas and Schlenoff it was concluded that amount of polymer deposited was controlled by the highest (rather than the least) salt concentration in polymer-containing solution to which the surface is exposed during a cycle. A thin layer, having been deposited from low salt concentration, is able to reconfirm to accommodate more polymers, but once adsorbed, polymer does not desorp [26].
It has been suggested that a negatively charged electrostatic barrier prevents the approach of additional polymer [34]. This argument has been used for single layer adsorption of polyelectrolyte [42]. A gradually increasing amount of charged polyelectrolyte with time is certainly consistent with such a barrier. However, at these high salt concentrations the electrostatic interaction within the polyelectrolyte should be sufficiently screened for the polymer to behave as though it were neutral. This repulsion, an excludedvolume effect, would probably be coupled with slow conformational changes on the surface or within the top few layers. Such slow rearrangements are well known in polyelectrolyte adsorption [44,45].
The literatures on polyelectrolyte multilayers contain a wide range of estimates (seconds to hours) of the time required to form a layer. The formation of a layer is probably limited by mass transport of polymer to the surface. The remainder will be added under a slower regime of permeation through surface polymer and rearrangements.
Excess charge
The key to polyelectrolyte multilayer propagation is surface charge reversal [3]. Each deposition step must leave the surface primed for the next immersion in oppositely charged polymer. This phenomenon is non intuitive: it has been generally assumed that polyelectrolyte associations have a 1:1 charge stoichiometry [34]. The addition of polymer is irreversible on the whole molecule scale, but mobility of short stretches of polymers must occur in order to accommodate more polymers. Additional theoretical treatments of overcompensation (overcharging) are now emerging.
Experiments with the surface forces [46-50], as well as the electrophoresis of multilayer-coated particles [51], have suggested that unpaired (uncomplexed) segments of the oppositely charged polymer coexist with the surface majority polymer.
Temperature
Polyelectrolyte adsorption to an oppositely charged interface is determined by electrostatic and secondary interaction. Since polyelectrolytes precipitate at elevated temperatures, the secondary interactions are presumably temperature dependent. Since on temperature increase the polymer-solvent interaction increases and more polyelectrolyte adsorb onto oppositely charged interface. This idea was verified for PAH/PSS films adsorbed from 1 M KCl aqueous solution at temperatures between 5 and 40° approaching the precipitation temperature of PSS at 60o. Indeed, the film thickness increases continuously with the adsorption of solution temperature; the changes amount to 20-40% (depending on salt condition) [52].
Different Type Of Umcs
Different types of UMCs are polyanion/polycation UMCs, polysaccharide UMCs, polyelectrolyte/ dendrimer UMCs, inorganic/organic hybrid multilayer ultra thin hollow spheres, lipid/polyelectrolyte UMCs and surface modified polyelectrolyte capsules as follows
Polyanion/polycation UMCs
A method for preparing multilayer ultra thin organic films by the consecutive deposition of oppositely charged polyelectrolyte from dilute aqueous solution on to charged solid support was introduced by Decher et al [2,3].
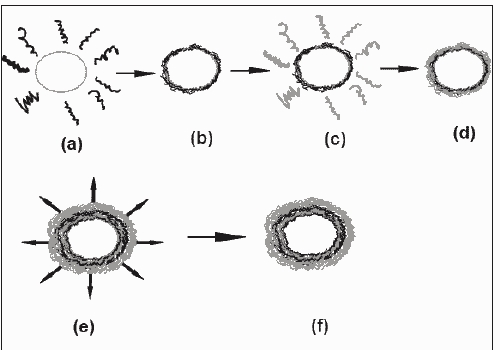
Micronized polyelectrolyte shells of PSS and PHA with the thickness ranging from a few to tens of nanometers have been produced by colloid template. Consecutively polyelectrolyte adsorption is followed by decomposition of templating core. Core used was weakly cross linked melamine formaldehyde (MF) colloidal particles [9]. These particles, capable to produce hollow capsules by decomposition in aqueous media at pH values below 1.6 are shown in fig. 1.
PSS/ PDADMAC capsules derived from MF colloidal particles can be prepared with a yield of unbroken capsules higher than 90% by the layer-by-layer adsorption technique followed by decomposition of the templated MF cores by exposing the coated particles to pH 1.1 HCl solutions. A membrane filtration technique was employed to consecutively adsorb PSS (negatively charged polyelectrolytes) and positively charged (PDADMAC) onto MF particles. The adsorption of polyelectrolytes was conducted in 0.5 M NaCl solution for 5 min followed by three washings in H2O. Then, the respective oppositely charged polyelectrolyte species was added. After the desired number of layers was assembled, the coated particles were treated in HCl solution (pH 1.1) to decompose the MF cores. The produced MF core degradation products and excess HCl were washed off until a neutral pH was established by filtration with gentle agitation. The outermost layer in this study is always the positively charged PDADMAC [53,54].
Polysaccharide UMCs
The polysaccharide multilayer encapsulation of ibuprofen crystals were accomplished by the consecutive adsorption of oppositely charged polyelectrolytes using centrifugation technique. In each experiment ibuprofen crystals were alternatively dispersed in chitosan or dextran sulphate solution. The opposite charged polysaccharides were allowed to adsorb for 10-15 min, while occasionally being shaken. The dispersion was then centrifuged at 10,000 rpm for 5 min. The supernatant was removed and particles were redispersed in deionzed water. The washing was repeated thrice before the next polyelectrolyte solution was added. The process was continued until desired coating was reached. Capsules were made of five pairs or either chitosan/dextran sulphate or chitosan/sodium corboxymethylcellulose [11].
Polyelectrolyte/dendrimer UMCs
Dendrimer coated particles prepared by using colloid templates are especially attractive for drug targeting and sustained release application as well as for separation process and also nanoreactor films made by dendrimers allows the entrapment of various species within them.
Khopade and Caruso demonstrated the successful layer-by-layer formation and subsequent dye loading of polyelectrolyte/dendrimer multiplayer films constructed on planar substrate as well as on colloids. They reported a versatile layer-by-layer approach to prepare poly (styrene sulfonate) (PSS)/ fourth generation poly (amidoamine) dendrimer (4G PAMAM) multiplayer films of defined thickness and composition under physiological salt concentration by exploiting electrostatic interactions. They first prepared the films on planar support to elucidate the factors that govern the formation of stable films and then constructed the PSS/4G PAMAM films on colloid substrate [12].
To prepare ultra thin polyelectrolyte/dendrimer coatings on colloids, PSS/4G PAMAM multilayers were adsorbed on the surface of particles using the conditions employed for the planar support and by applying the layer-by-layer assembling protocol to colloids [56-59] (no drying step was employed after deposition of each layer). Stepwise growth was confirmed by the successful recharging of the particle surface with each deposition cycle. The ζ-potential of the particles alternated between -30 mV (PSS) and +45 mV (4G PAMAM) with each coating step suggested multilayer growth occurred on the particles [12].
Complexes of 4G PAMAM and a long chain polyelectrolyte (e.g. PSS) are significantly different from those made of linear oppositely charged polyelectrolytes, in that the dendritic core offers the possibility of selectively entrapping guest molecules 4,5-carboxyflurescim (CF), a model acidic substance, was entrapped in PSS/4G PAMAM multiplayer. Since CF does not bind to PSS, the loading observed is due to the interaction of CF with the dendrimer.
As the coated colloids extends, hollow polyelectrolyte/ dendrimer capsules were obtained after removal of the core. The thin and highly porous capsules were fragile during the core removal procedure; therefore they prepared UMCs with an outer coat of PSS/PAH/PSS. The capsules obtained were stable and consisted of folds due to drying effects, displaying a similar morphology to other hollow capsules made of linear poly electrolytes [58]. These capsular colloids represent interesting freestanding systems for loading and controlled release, as they can exhibit elastic properties, similar to biological cells [9,60,61].
Inorganic/organic hybrid multilayer ultra thin hollow spheres
Caruso, et al., demonstrated that the inorganic/ organic hybrid self-assembly technique, when applied to produce composite SiO2 nanoparticle–polymer multilayers on colloids, coupled with removal of the core and (optionally) the polymer, provides a successful pathway for fabricating inorganic and organic hybrid hollow sphere in the submicrometer to micrometer size range. Important advantages of this method of fabricating hollow spheres are: (i) the thickness of the hollow sphere walls can be readily controlled by varying the number of deposition cycles. (ii) The size and shape of the spheres produced are determined by the dimensions of the templating colloid employed; (iii) the method is generally applicable to a wide variety of charged inorganic particles, thereby making possible the production of various inorganic (such as ZiO2 and TiO2) and composite (magnetic nanoparticle and SiO2 or TiO2) hollow spheres [57].
Lipid/polyelectrolyte UMCs
The combination of lipid and polyelectrolyte layers may be useful for understanding the principles of the interaction of membranes with biopolymers, such as proteins, DNA, or carbohydrates. Moya, et. al., reported that lipids can adsorb on to colloidal particles coated with polyelectrolytes and polyelectrolyte capsules [62]. Charged lipids, such as dipalmitoyldiphosphatidic acid (DPPA), form bilayers while zwitterionic lipids, such as dipalmitoyldiphosphotidyl choline (DPPC), may adsorb more than one bilayer. The lipid assembling can be performed either by adsorption of lipid vesicles on to colloidal particles or by adsorption of lipids from a methanol solution during a gradual exchange of methanol for water. In the case of polyelectrolyte capsules the coating with lipids provides a new means to control the permeability of the capsules, hence encouraging their use for slow release of polar substance. In comparison to liposomes or vesicles, the lipid/polyelectrolyte composite are more stable systems with a size predefined by the size of the polyelectrolyte capsule [62].
The lipid layers adsorbed on the supporting skeleton of a polyelectrolyte matrix represent an interesting model system, which could be used for biophysical studies. They have a high mechanical stability together with determined size and shape. Reconstruction of various biological functions can be envisaged by the incorporation of protein functionalities into the adsorbed lipid layer.
Surface modified polyelectrolyte capsules
Moya et al. concluded that polyelectrolyte capsules consist of PSS/PAM layers when coated with an additional layer of lipid, there is a remarkable reduction in the permeability of the capsule wall.
This provides a new means to control the permeability of capsules, hence encouraging the use as a container for slow release of polar substance [62]. Also surface modifications of polyelectrolyte multilayer-coated particles can be done for biological applications. The surface modified (PEGylated) PSS/4G PAMAM UMCs reduces the adhesion to the biological cell surface (macrophage cell line TPH-1) [63].
Conclusion
UMCs application in pharmaceutical fields is just in the incipient stage. The use of UMCs in the design of new drug delivery system and devices is used in closely related fields such as protein delivery, gene delivery and diagnostic sensors, which receives increasing attention. The concept of electrostatically driven assembly of multilayer structures allows for the incorporation of a wealth of different materials like drugs (ciprofloxacin hydrochloride [7], ibuprofen, furosemide [11], nifedipine, and theophylline), dyes (flurorescein particles [36,58], pyrene), proteins (myoblogin, cytochrome P450cam), and polynucleotides like DNA [64].The incorporation of proteins in multilayer films may lead to the application of UMCs as biosensors or in biotechnology; they may even provide the base for new developments in multi step chemical catalysis. UMCs appear to be a promising tool in the drug delivery for the pharmaceutical scientist but exclusive research work is needed before any meaningful conclusion could be drawn. The issues relating to biocompatibility, and toxicity of UMCs should also be seriously addressed.
References
- Jain NK. Controlled and Novel Drug Delivery. 2 nd ed. CBS Publishers: New Delhi; 1998.
- Decher G, Hong JD, Schimitt J. Build up of ultra thin multilayer films by a self-assembly process. Consecutively alternating adsorption of anionic and cationic polyelectrolytes on charged surfaces. Thin Solid Film 1992;211:831-835.
- Decher G. Fuzzy nanoassemblies: Towards layered polymeric multicomposites. Science 1997;277:1232-7.
- Onda K, Lvov Y, Ariga K, Kumitake T. Sequential action of glucose oxidase and peroxidase in molecular films assembled by layer-by-layer alternate adsorption. Biotechnol Bioengg 1996;51:163-7.
- Schnitt J, Grunewald T, Decher G, Pershan P, Kjaer K, Lesche M. Internal structure of layer-by-layer adsorbed polyelectrolyte films: A neutron and X-ray reflexitivity study. Macromolecules 1993;26:7058-63.
- Ariga K, Lvov Y, Kunitake T. Assembling alternate dye polyion molecular films by electrostatic layer-by-layer adsorption. J Am Chem Soc 1997;119:2224.
- Bhadra D, Gupta G, Bhadra S, Umamaheshwari RB, Jain NK. Multicomposite ultrathin capsules for sustained ocular delivery of ciprofloxacin hydrochloride. J Pharm Pharmacol Sci 2004;7:241-51.
- Lvov Y, Ariga K, Ichinose I, Kunitake T. Assembly of multicomponent protein films by means of electrostatic layer-by-layer adsorption. J Am Chem Soc 1995;117:6117-23.
- Donth E, Sukhorulov G, Caruso F, Davis S, Mohwold H. Novel hollow polymer shells by colloidal template assembly of polyelectrolytes. Angew Chem Int Ed 1998;37:2202-5.
- Sukhorukov GB, Donath E, Lichtenfield H, Knippel E, Knippel M, Budde A, et al . Layer-by-layer self assembly of polyelectrolytes on colloidal particles. Colloids Surface-A Physiochem Eng Aspects 1998;137:253-66.
- Qiu X, Leporatti S, Donath E, Mohwald H. Studies on the drug release properties of polysaccharide multilayer encapsulated ibupropen microparticles. Langmuir 2001;17:5375-80.
- Khopade X, Caruso F. Electrostatically assembled polyelectrolyte/dendrimer mutilayer films as ultrathin nanoreservoirs. Nanoletters 2002;2:415-8.
- Sukhorukov GB, Mantrel MM, Petrov AI, Shabarchina LI. Multilayer films containing immobilized nuclecie acids. Their structure and possibilities in biosensor application. Biosens Bioelectron 1996;11:913.
- Sukhorukov GB, Brumen M, Donath E, Mohwald H. Hollow polyelectrolyte shells: Exclusion of polymers and donnan equilibrium. J Phys Chem B 1999;103:6434-40.
- Ferreira M, Rubner MF, Hscich BR. Polyelectrolyte multilayers, an overview. Master Res Soc Symp Proc 1994;328:119-24.
- Lvov Y, Ariga K, Ichinose I, Kunitake T. Formation of ultrathin multilayer and hydrated gel from montmorillonite and linear polycations. Langmuir 1996;12:3038-44.
- Keller SW, Johnson SA, Brigham ES, Yonemoto EH, Mallouk TE. Photo-induced charge separation in multilayer thin films grown by sequential adsorption of polyelectrolytes. J Am Chem Soc 1995;177:12879-80.
- Decher G. Templating self-assembly and self organization savage. In : Hosseini JP, editors. Chapter 14, Vol. 9, Pergamon Press: Oxford;1996. p. 507-14.
- Laschewsky A. Photophysical behavior of a new gemini surfactant in neat solvents and in micellar environments. Eur Chem Chron 1997;2:13-8.
- Schonhoff M. Self-assembled polyelectrolyte multilayers. Curr Opin Colloid Interface Sci 2003;8:86-95.
- Jaber JA, Schlenoff JB. Mechanical properties of reversibly cross-linked ultrathin polyelectrolyte complexes. J Am Chem Soc 2006;128:2940-7.
- Schlenoff JB, Ly H, Li M. Charge and mass balance in polyelectrolyte multilayers. J Am Chem Soc 1998;120:7626-34.
- Decher G, Schmitt J. Fine-tuning of the film thickness of ultrathin multilayer films composed of consequently alternating layers of anionic and cationic polyelectrolyte. J Prog Colloid Polym Sci 1992;89:160-165.
- Arys X, Jonas A, Laschewsky A, Legras R, editors. Supramolecular polymers. Dekker; New York; 2003.
- Losche M, Schmitt J, Decher G, Bouwman WG, Kjaer K. Detailed structure of molecularly thin polyelectrolyte multilayer films on solid substrates as revealed by neutron reflectometry. Macromolecules 1998;31:8893-906.
- Dubas ST, Schlenoff JB. Factors controlling the growth of polyelectrolyte multilayers. Macromolecules 1999;32:8153-60.
- Yoo D, Shiratori SS, Rubner MF. Controlling bilayer composition and surface wett ability of sequentially adsorbed multilayers of weak polyelectrolytes. Macromolecules 1998;31:4309-18.
- Schlenoff JB, Ly H, Li M. Charge and mass balance in polyelectrolyte multilayers. J Am Chem Soc 1998;120:7626-34.
- Fleer GJ, Stuart CM, Scheutjens JM, Cosgrove T, Vincent B, editors. Polymers at Interfaces. Chapman and Hall: London; 1993.
- Van de Steeg HG, Stuart CM, de Keizer A, Bijsterbosch BH. Polyelectrolyte adsorption: A subtle balance of forces. Langmuir 1992;8:2538-46.
- Schinhoff JB, Dubas ST. Mechanism of oplyelectrolyte multilayaer growth: Charge our compensation and distribution. Macromolecules 2001;34:592-8.
- Gao C, Leporatti S, Moya S, Donath E, Mohwald H. Stability and mechanical properties of polyelectrolyte capsules obtained by stepwise assembly of poly(styrenesulfonate sodium salt) and Poly(diallyldimethyl ammonium) Chloride onto melamine resin particles. Langmuir 2001;17:3491-5.
- Lowack K, Helm CA. Molecular mechanisms controlling the self-assembly process of polyelectrolyte multilayers. Macromolecules 1998;31:823-33.
- Dautzenberg H, Jaeger W, Kotz J, Philipp B, Seidel CH, Stscherbina D, editors. Polyelectrolytes: Formation, Characterization and Application. Munich: Hanser; 1994.
- Lvov Y, Ariga K, Onda M, Ichinose I, Kunitake T. A careful examination of the adsorption step in the alternate layer-by-layer assembly of linear polyanion and polycation. Colloids Surf A 1999;146:337-46.
- Caruso F, Donath E, Mohwald H. Influence of polyelectrolyte multilayer coatings on f φrster resonance energy transfer between 6-carboxyfluorescein and rhodamine b-labeled particles in aqueous solution. J Phys Chem B 1998;102:2011-6.
- Antipov AA, Sukhorukov GB, Donath E, Mohwald H. Sustained release property of polyelectrolyte multilayer capsules. J Phys Chem B 2001;105:2281-4.
- Chia SM, Wan ACA, Quek CH, Mao HQ, Xu X, et al. Multi-layered microcapsules for cell encapsulation. Biomat 2002;23:849-56.
- Lourenco JM, Ribeiro PA, Botelho do Rego AM, Braz FM, Moutinho AM, Raposo M. Counterions in poly(Allylamine hydrochloride) and poly(Styrene sulfonate) layer-by-layer films. Langmuir 2004;14:8103-9.
- Lvov Y, Ariga K, Onda M, Ichinose I, Kunitake T. A careful examination of the adsorption step in the alternate layer-by-layer assembly of linear polyanion and polycation. Colloids Surf A 1999;146:337-46.
- Losche M, Schmitt J, Decher G, Bouwman WG, Kjaer K. Detailed structure of molecularly thin polyelectrolyte multilayer films on solid substrate as revealed by neutron reflectrometry. Macromolecules 1998;31:8893-906.
- Stuart CM, Daillant J, Guenoun P, Marques C, Muller P, Tran Thanh Van J, editors. Frontier: Short and Long Chains at Interfaces. Gif-sur-Yvette: France; 1996.
- Gobel JG, Besseling NA, Cohen Stuart MA, Poncet C. Adsorption of hydrophobically modified polyacrylic acid on a hydrophobic surface: Hysteresis caused by an electrostatic adsorption barrier. J Colloid Interface Sci 1999;209:129-35.
- Meadows J, Williams PA, Garvey MJ, Harrop RA, Phillips GO. Enhanced polyelectrolyte adsorption. Colloids Surf 1988;32:275-88.
- Pefferkorn E, Jean-Chronberg AC, Varoqui R. Conformational relaxation of polyelectrolytes at a solid-liquid interface. Macromolecules 1990;23:1735-41.
- Marshal RJ, Gronbech-Jensen N, Fitzsimmons MR, Lutt M, Li D. Theoretical and experimental adsorption studies of polyelectrolytes on an oppositely charged surface. J Chem Phys 1999;110:2219.
- Nordmeier E, Beyer P. Synthesis and characterisation of dextran and pullulan sulphate. J Polym Sci part B: Polym Phys Ed 1999;37:335-41.
- Park SY, Bruinsma RF, Gelbart WM. Spontaneous overcharging of macro-ion complexes. Europhys Lett 1999,46:454-60.
- Joanny JF. Polyelectrolyte Adsorption and Charge Inversion. Eur Phys JB 1999;9:117-22.
- Solis FJ, Cruz IMO. Surface-induced layer formation in polyelectrolytes. J Chem Phys 1999;10:115-7.
- Donath E, Walther D, Shilov VN, Knippel E, Budde A, Lowack et al. Nonlinear bairy layer theory of electrophoretic fingerprinting applied to consecutive layer-by-layer polyelectrolyte adsorption onto charged polystyrene latex particles. Langmuir 1997;13:5294-305.
- Buscher K, Graf K, Ahrens H, Helm CA. Influence of adsorption conditions on the structure of multilayers. Langmuir 2002;18:585-91.
- Gao C, Leporatti S, Moya S, Donath E, Mohwald H. Stability and mechanical properties of polyelectrolyte capsules obtained by stepwise assembly of poly(styrenesulfonate sodium salt) and Poly(diallyldimethyl ammonium) chloride onto melamine resin particles. Langmuir 2001;17:3491-5.
- Voigt A, Lichtenfeld H, Sukhorukov GB. Zastrow H, Donath E, Baumler, et al. Membrane filtration for microencapsulation and microcapsules fabrication by layer-by-layer polyelectrolyte adsorption. Ind Eng Chem Res 1999;38:4037-43.
- Schonhoff M. Self-assembled polyelectrolyte multilayers, Curr Opin Colloid Interface 2003;8:86-95.
- Chorely C, Pouliquen D, Lucent L, Jallet PJ. Development of superparamagnetic nanoparticles for MRI: Effect of particle size, charge and surface nature on biodistribution. Microencapsulation 1996;13:245-55.
- Caruso F, Caruso RA, Mohwald H. Nanoengineering of inorganic and hybrid hollow spheres by colloidal templating. Science 1998;282:1111-4.
- Caruso F, Lichtenfeld H, Donath E, Mohwald H. Investigation of electrostatic interaction in polyelectrolyte multilayer films: Binding of anionic florescent probes to layers assembled onto colloids. Macromolecules 1999;32:2317-28.
- Caruso F. Nanoenginerring of particle surface. Adv Mater 2001;13:11.
- Shuler C, Caruso F. Decomposable hollow biopolymer capsules. Biomacromolecules 2001;2:921-6.
- Pastoriza-Santos I, Scholer B, Caruso F. Core-shell colloids and hollow polyelectrolyte capsule based on diazoresins. Adv Functional Mater 2001;11:122-8.
- Moya S, Donath E, Sukhorukov GB, Auch M, Baulmer H, Lichtenfeld et al. Lipid coating on polyelecrolyte surface modified colloidal particles and polyelectrolyte capsules. Macromolecules 2000;33:4538-44.
- Khopade A, Caruso F. Surface-modification of polyelectrolyte mutilayer-coated particles for biological applications. Langmuir 2003;19:6219-25.
- Pei R, Cui X, Yang X, Wang E. Assembly of alternating polycation and DNA multilayer films by electrostatic layer-by-layer adsorption. Biomacromolecules 2001;2:463-8.