- *Corresponding Author:
- D. Liu
Department of Pharmacy, Tongji Hospital, Tongji Medical college, Huazhong University of Science and Technology, Hubei Wuhan 430033, China
E-mail: ld2069@outlook.com
| Date of Submission | 22 September 2016 |
| Date of Revision | 09 January 2017 |
| Date of Acceptance | 10 May 2017 |
| Indian J Pharm Sci 2017;79(4):527-535 |
Abstract
Adverse event reports related hepatoprotective drugs are increasing. This has important public health implications. Our study aims to investigate the safety of hepatoprotective drugs by combining safety reports with drug utilization data from 2012 to 2014. Utilization data were retrieved from the hospital drug information network of Yangtze River basin and expressed as defined daily doses per 10 000 inhabitants per day. Safety data were collected from the Hubei Adverse Event Reporting System and analyzed according to patients’ sex, age, occurrence time, route of administration, involved organs or systems, and so on. The relationship between utilization and adverse drug events was used to evaluate the safety of hepatoprotectives by graphical analysis with histogram plots. For the utilization, both consumption sum and dose per inhabitants per day increased from 2012 to 2014. Of the 21 hepatoprotectives, 52.38% were chemical drugs, which accounted for 78.95% of total expenditure and 51.64% of total dose per inhabitants per day. The top three hepatoprotectives were glycyrrhizin, hepatocyte growth-promoting factors, and marine. For the safety, chemical drugs were responsible for 673/916 adverse drug events (73.47%). The gastrointestinal system was most frequently damaged (331, 29.82%), followed by the skin and its appendages (324, 29.19%). Irrational use and intravenous administration were associated with an increased risk of adverse drug events, Therefore, surveillance and educational strategies should be strengthened to promote the safety of hepatoprotective drugs. Of the 21 hepatoprotectives, glycyrrhizin, Heluoshugan, and Bicyclol were preferable for clinical hepatoprotection, whereas Yinzhihuang, Kuhuang, and ribonucleic acid should be avoided. This parallel approach through spontaneous reporting and drug utilization analyses provided valuable information for the safety of hepatoprotectives, and this synergy should be encouraged to support future pharmacovigilance activities.
Keywords
Adverse drug events, drug utilization, postmarketing drug surveillance, Pharmacoepidemiology
Hepatoprotective drugs, also referred to as hepatoprotectives, can improve liver function, promote the regeneration of liver cells and enhance liver detoxification, thus having been widely used to treat liver diseases [1,2]. Chinese expert consensus on the prevention and treatment of hepatic inflammation and injury [2] classifies hepatoprotectives as antiinflammatory drugs, liver cell membrane stability fixers, detoxicants, antioxidants and choleretics. Up to now, dozens of hepatoprotectives have been available in clinical practice. Faced with this wide array of choices [3-8], it is imperative for clinicians to be familiar with the strengths and weaknesses of these agents to optimize therapy. However, there are still limited data on the safety of hepatoprotectives, particularly in special populations such as the very young and the elderly. Therefore, promoting the study of hepatoprotectives in this area is of great significance.
This retrospective analysis investigates the utilization and safety of 21 hepatoprotectives commonly used in hospitals of Hubei Province in China, which are composed of 11 Chinese herbal medicine and 10 chemical drugs. To fully understand the manifestations and factors associated with the safety of different hepatoprotectives, we analysed by combining reported adverse drug events (ADEs) from the Hubei Adverse Event Reporting System (HAERS) with drug utilization data from 2012 to 2014. This study could be very useful to improve the health system with regard to financial aspects and safe evaluations, and we hope that our findings can provide guidance for clinicians, and thus help to avoid hepatoprotectives-associated ADEs in the future.
Materials and Methods
The drug utilization and ADE data of hepatoprotectives were obtained from Yangtze River hospital drug information network and HAERS, respectively. The two databases included drug information of 34 hospitals in Hubei Province. Among them, 22 were tertiary care hospitals with more than 500 beds, 10 were secondary ones with 200-500 beds, and 2 were primary level hospitals with less than 200 beds. The data concerning drug utilization contained drug name, specifications, packaging, quantity, amount, etc. The ADE data included patients’ name, sex, age, race, ADE history, ADE name, ADE process description, drug information, relevance evaluation, ADE analysis, etc. All reports related to the 21 hepatoprotectives notified between January 1, 2012, and December 31, 2014, to HAERS, excluding those with missing items and errors such as child’s birth date, weight, dosage and units of drug, ADE name and other important items, were selected and evaluated according to patients’ sex, age, route of administration, involved organs or systems, and possible ADEs.
Defined daily dose (DDD) and defined daily dose cost (DDDc)
The drug utilization data were collected and analysed using the DDD methodology [9]. The following formula was used to calculate DDDs, DDDs = strength of dosage form×quantity/WHO-assigned DDD for the product. The authors reported the mean DDDs per 10 000 d (now referred to as DID). DDDc, which was calculated by dividing the total cost with DDDs, showed the average daily cost of drug.
Definition of ADEs, adverse drug reactions (ADRs), hypersensitivity and allergy
ADEs are untoward and unintended events arising from the use or misuse of drugs, whether or not they are drug-related. ADRs are defined as ‘noxious and/ or unintended responses to medication, which occur at doses normally used for prophylaxis, diagnosis or therapy of disease or for modification of physiological function’. Hypersensitivity is described as ‘objectively reproducible symptoms or signs initiated by exposure to a defined stimulus at a dose tolerated by normal persons’. Allergy is a hypersensitivity reaction initiated by specific immunologic mechanisms.
Nature of ADEs
ADEs are categorized as new, serious or general [10]. ADEs are considered as new when: not stated in drug instructions; inconsistent in the nature, extent, consequences or frequency compared with those described in the drug instructions, or even worse. ADEs are considered as serious when: fatal; life-threatening; carcinogenic, teratogenic or birth-defective; permanently/significantly disabling or organ-damaging; hospitalization is required or prolonged; intervention is required to prevent permanent impairment or damage. ADEs are considered as general for the remaining.
Causality of ADEs
In order to assess the likelihood that ADEs were caused by hepatoprotectives, a causality rating was assigned to each drug using the modified algorithm [11]. Briefly, five questions as follows were asked: does the occurrence of ADE have a reasonable time relationship to the use of the drug (for time-related reactions)? Does the pattern of ADE fit one of the known adverse reactions of the suspected drug? Does the reaction abate or disappear as the dose of the suspected drug is reduced or withdrawn (for dose-related reactions)? Does the adverse event recur when the same drug was used again (rechallenge)? Can the occurrence of adverse event be explained with combined agents, underlying disease and other chemicals? Based on their answers (Table 1), ADEs causality was determined as definite, probable, possible, possible irrelevant, to be evaluated and unevaluated [12].
| Classification | I | II | III | IV | V |
|---|---|---|---|---|---|
| Definite | Yes | Yes | Yes | Yes | No |
| Probable | Yes | Yes | Yes | No rechallenge | No |
| Possible | Yes | No | Unknown | No rechallenge | Unknown |
| Possible irrelevant | No | No | Unknown | No rechallenge | Unknown |
| To be evaluated | More data are essential for a proper assessment or the additional data are being examined | ||||
| Unevaluated | Information is insufficient or contradictory and cannot be supplemented or verified | ||||
Table 1: Causality evaluation based on answers of five questions
Patient characteristics
Patients were subdivided into nine age groups: infants and children (0-10 y), adolescents (11-20 y), young adults (21-30 y), adults (31-40 y), older adults (41-50 y), elderly adults (51-60 y), geriatric (61-70 y), older geriatric (71-80 y) and very elderly geriatric (over 81 y).
Evaluation of safety
The following formulas were used to calculate the percentage of utilization frequency (%DDDs) and the percentage of ADEs, respectively: %DDDs = DDDs of each hepatoprotective/total DDDs; %ADEs = ADEs caused by each hepatoprotective. The relationship between utilization and ADEs was used to evaluate the safety of hepatoprotectives by graphical analysis with histogram plots [(ADEs)/(DDDs)]. Higher %DDDs and lower %ADEs meant the drugs were safer.
Statistical analysis
Data were statistically analysed using the SPSS 11.5 statistical software (SPSS Inc., Chicago, IL, USA). The chi-square test of crosstabs was used to examine associations between categorical variables All P values were 2-tailed, and differences were considered statistically significant when P-values were <0.05.
Results and Discussion
The resident population in Hubei Province showed a slight increase from 2012 to 2014, and there were 57.79, 57.99 and 58.16 million people, respectively in 2012, 2013 and 2014. The proportion of the elderly population exceeded 10% in 2013 for the first time, and this represented an important challenge to the health system. In this study, we found that both consumption sum (increased by 20.17% in 2013, and 9.08% in 2014) and DID (increased by 31.74% in 2013, and 35.99% in 2014) of the hepatoprotectives kept increasing from 2012 to 2014, and the growth rate was far greater than that of the population. This situation may be associated with the ageing population. Of the 21 hepatoprotectives, 52.38% were chemical drugs, which accounted for 78.95% of total expenditure and 51.64% of total DID. Magnesium isoglycyrrhizinate ranked first in consumption sum, followed by ornithine aspartate and ribonucleic acid. Glycyrrhizin, hepatocyte growthpromoting factors and marine had the highest DID, which were 606.25, 576.77, and 508.15, respectively. Bifendate was, as suggested by DDDc, the cheapest (US$ 0.06 daily), while ribonucleic acid was the most expensive (US$ 44.41 daily) (Table 2).
| Category | Drug | Consumption sum/(US$ 10 000) | % | Order | DDDs (per 10 000 d) |
Order | DDDc (US$/d) | Order |
|---|---|---|---|---|---|---|---|---|
| Chemical drug | Hepatocyte growth-promoting factors | 331.75 | 2.92 | 8 | 576.77 | 2 | 0.58 | 19 |
| Glycyrrhizate monopotassium | 36.76 | 0.32 | 19 | 45.22 | 16 | 0.81 | 18 | |
| Diammonium glycyrrhizinate | 733.23 | 6.46 | 7 | 279.80 | 5 | 2.62 | 9 | |
| Ribonucleic Acid | 1544.95 | 13.62 | 3 | 34.79 | 17 | 44.41 | 1 | |
| Bifendate | 1.76 | 0.02 | 21 | 140.78 | 7 | 0.01 | 21 | |
| Polyene phosphatidylcholine | 1064.84 | 9.39 | 4 | 317.63 | 4 | 3.35 | 8 | |
| Metadoxine | 12.88 | 0.11 | 20 | 3.71 | 21 | 3.47 | 7 | |
| Ornithine aspartate | 1806.83 | 15.92 | 2 | 155.85 | 6 | 11.59 | 5 | |
| Bicyclol | 177.71 | 1.57 | 13 | 90.83 | 13 | 1.96 | 12 | |
| Ademetionine | 834.30 | 7.35 | 6 | 64.75 | 14 | 12.89 | 4 | |
| Magnesium isoglycyrrhizinate | 2413.31 | 21.27 | 1 | 138.25 | 8 | 17.46 | 3 | |
| TCM | Anluohuaxian | 296.16 | 2.61 | 9 | 134.31 | 9 | 2.21 | 10 |
| Fuzhenghuayu | 259.84 | 2.29 | 10 | 127.28 | 11 | 2.04 | 11 | |
| Glycyrrhizin | 1044.20 | 9.20 | 5 | 606.25 | 1 | 1.72 | 14 | |
| Heluoshugan | 137.70 | 1.21 | 14 | 120.14 | 12 | 1.15 | 17 | |
| Hujuyigan | 58.81 | 0.52 | 17 | 31.04 | 18 | 1.89 | 13 | |
| Marine | 61.12 | 0.54 | 16 | 508.15 | 3 | 0.12 | 20 | |
| Kuhuang | 53.84 | 0.47 | 18 | 7.35 | 19 | 7.33 | 6 | |
| Shugan | 211.08 | 1.86 | 11 | 5.39 | 20 | 39.13 | 2 | |
| Silibin Meglumine | 188.43 | 1.66 | 12 | 133.83 | 10 | 1.41 | 15 | |
| Yinzhihuang | 76.37 | 0.67 | 15 | 57.31 | 15 | 1.33 | 16 | |
| Total | 11345.87 | 100 |
Table 2: Consumption of hepatoprotectives based on generic name from 2012-14
Of the 916 ADEs, 570 (62.23%) were males and 346 (37.77%) were females, with the sex ratio of 1.65:1. The percentages of patients with reported ADEs varied among the nine age groups. The youngest case was 3 d old, and the oldest was 91 y old. The most commonly affected age group was 41 to 70 (65.83%). However, there was no significant difference between the age and sex by chi-squre test (P=0.56). Sex and age distribution of ADEs is shown in Table 3.
| Age (y) | Male | Female | Total | % | ||
|---|---|---|---|---|---|---|
| Number of cases | % | Number of cases | % | |||
| 0-10 | 18 | 1.97 | 10 | 1.09 | 28 | 3.06 |
| 11-20 | 9 | 0.98 | 7 | 0.76 | 16 | 1.75 |
| 21-30 | 49 | 5.35 | 26 | 2.84 | 75 | 8.19 |
| 31-40 | 78 | 8.52 | 37 | 4.04 | 115 | 12.55 |
| 41-50 | 137 | 14.96 | 95 | 10.37 | 232 | 25.33 |
| 51-60 | 140 | 15.28 | 84 | 9.17 | 224 | 24.45 |
| 61-70 | 96 | 10.48 | 51 | 5.57 | 147 | 16.05 |
| 71-80 | 34 | 3.71 | 25 | 2.73 | 59 | 6.44 |
| >80 | 9 | 0.98 | 11 | 1.20 | 20 | 2.18 |
| Total | 570 | 62.23 | 346 | 37.77 | 916 | 100.00 |
Table 3: Sex and age distribution in ADEs
Chemical drugs were responsible for 673/916 ADEs (73.47%). In particular, 210 episodes (22.93%) were associated with diammonium glycyrrhizinate, followed by ornithine aspartate (172, 18.78%), and the most frequently manifested were nausea or vomiting, rash or pruritus, palpitation or chest tightness. In traditional Chinese medicines (TCMs), Yinzhihuang had the worst outcomes (173, 18.89%) and the most frequently manifested were anaphylactoid reaction, nausea or vomiting, rash or pruritus. The constituent ratios and main clinical manifestations of ADEs caused by each individual hepatoprotective are listed in Table 4. ADE occurrence times fluctuated widely, with the shortest in 1 min after intravenous drip and the longest in 24 d of continuous administration. Regardless of traditional Chinese medicine (TCM) or chemical drugs, ADEs tended to occur the first time the medication was given, usually in the first 30 min of use. Notably, 76.9% of serious adverse reactions occurred within 30 min after administration. ADEs with time distribution are shown in Table 5. The gastrointestinal system was most frequently damaged (331, 29.82%), followed by damages of the skin and its appendages (324, 29.19%) and systemic ones (152, 13.69%). The organs or systems involved and the main clinical manifestations of ADEs are listed in Table 6.
| Category | Drugs involved | Number of ADEs | % | Main clinical manifestations |
|---|---|---|---|---|
| Chemical drug | Hepatocyte growth-promoting factors | 78 | 8.52 | Rash or pruritus (18), palpitation or chest tightness (9), fever of chills (7), dyspnoea (7), anaphylactoid reaction(6), phlebitis (5), dizziness (4), night sweats (4), nausea and vomiting (3), rash erythematous (3), flushing (2), anaphylactic shock (2), stomach discomfort (2), larynx oedema (2), anaesthesia local (2), pain (1), convulsions (1) |
| Glycyrrhizate monopotassium | 1 | 0.11 | Nausea or vomiting (2), constipation (1), mouth dry (1) | |
| Diammonium glycyrrhizinate | 210 | 22.93 | Nausea or vomiting (46), rash or pruritus (39), palpitation or chest tightness (19) dizziness (17), headache (15), anaphylactoid reaction (13), rash maculopapular (10), chills or fever (8), flatulence (6), abdominal pain (5), hypertension (4), mouth dry (4), dyspnoea(4), pain (2), appetite disorder (2), oedema (2), oedema periorbital (2), rash erythematous (2), tinnitus (1), hypocalcaemia (1), renal function abnormal (1), neuralgia (1), hyperuricemia (1), somnolence (1), stomach discomfort (1), hypotension (1), diarrhoea (1), facial oedema (1), | |
| Ribonucleic acid | 31 | 3.38 | Rash or pruritus (14), palpitation (4), dizziness (4), chest tightness (3), anaphylactoid reaction (2), anaphylactic shock (2), nausea or vomiting(1), pain (1), chills (1) | |
| Bifendate | 4 | 0.44 | nausea or vomiting(2), constipation (1), mouth dry (1) | |
| Polyene phosphatidylcholine | 85 | 9.28 | Rash or pruritus (15), palpitation or chest tightness (15), chills or fever (11), nausea or vomiting(9), anaphylactoid reaction(5), phlebitis (4), dizziness (4), rash erythematous (4), injection site pruritus (2), stomach discomfort (2), headache (2), dyspnoea(2), diarrhoea (2), anorexia (1), injection site pain (1), rash maculopapular (1), abdominal pain (1), agitation (1), hypotension (1), pain (1), dry cough (1) | |
| Metadoxine | 2 | 0.22 | Nausea or vomiting (2) | |
| Ornithine aspartate | 172 | 18.78 | Nausea or vomiting (76), palpitation or chest tightness (52), dizziness (11), chills or fever (8), rash or pruritus (5), headache (5), anaphylactoid reaction (4), abdominal pain (3), eructation (1), salivary gland enlargerment (1), asthenia (1), sweating increased (1), hypertension (1), dyspnoea (1), arthralgla (1), paraesthesia (1) | |
| Bicyclol | 1 | 0.11 | Nausea or vomiting (1) | |
| Ademetionine | 22 | 2.40 | Phlebitis (9), nausea or vomiting (2), anaphylactoid reaction(2), injection site pain (2), palpitation or chest tightness (2), rash (2), rash erythematous (1), flushing (1), headache (1) | |
| Magnesium isoglycyrrhizinate | 67 | 7.31 | Rash or pruritus (14), palpitation or chest tightness (12), nausea or vomiting (7), chills or fever (5), anaphylactoid reaction(4), rash erythematous (4), dizziness (2), anaphylactic shock (2), anasarca (2), peripheral oedema (2), oedema (2), injection site pruritus (2), injection site pain (1), rash maculopapular (1), flushing (1), phlebitis (1), night sweats (1), facial oedema (1), abdominal pain (1), hypoproteinaemia (1) | |
| TCM | Anluohuaxian | 6 | 0.66 | Nausea or vomiting (3), anaphylactoid reaction (2), diarrhoea (1) |
| Fuzhenghuayu | 2 | 0.22 | Rash (1), constipation (1) | |
| Glycyrrhizin | 3 | 0.33 | Headache (3) | |
| Heluoshugan | 1 | 0.11 | Diarrhoea or flatulence (1) | |
| Hujuyigan | 1 | 0.11 | Flatulence or insomnia (1) | |
| Marine | 13 | 1.42 | Nausea or vomiting (3), abdominal pain (3), rash(2), palpitation (1), injection site pruritus (1), fever (1), pruritus (1), chest tightness (1) | |
| Kuhuang | 35 | 3.82 | Rash (9), nausea or vomiting(6), fever (4), chills (3), anaphylactoid reaction(3), pain (2), anaphylactic shock (1), convulsions (1), diarrhoea (1), diarrhoea (1), dizziness (1), pruritus (1), palpitation (1), chest tightness (1) | |
| Shugan | 4 | 0.44 | Rash (2), nausea or vomiting (1), cough (1) | |
| Silibin Meglumine | 5 | 0.55 | Dizziness (3), rash (1), nausea or vomiting (1) | |
| Yinzhihuang | 173 | 18.89 | Anaphylactoid reaction (41), nausea or vomiting (38), rash or pruritus (36), fever (7), diarrhoea (7), chest tightness (6), rash maculopapular (5), palpitation (5), dyspnoea (3), pain (3), rash erythematous (3), stomach discomfort (3), larynx oedema (2), injection site pruritus (2), chills (2), dizziness (2), flatulence (2), fatigue (2), anaphylactic shock (1), coma (1), increased stool frequency (1), hypotension (1) | |
| Total | 916 | 100.00 |
Table 4: Constituent ratios and main clinical manifestation of each individual hepatoprotective
| Time | Chemical drug | TCM | Total | % | ||
|---|---|---|---|---|---|---|
| Number of cases | % | Number of cases | % | |||
| ≤5 min | 49 | 5.35 | 8 | 0.87 | 57 | 6.22 |
| 6-30 min | 216 | 23.58 | 79 | 8.62 | 295 | 32.21 |
| 31-60 min | 135 | 14.74 | 39 | 4.26 | 174 | 19.00 |
| 1-24 h | 84 | 9.17 | 59 | 6.44 | 143 | 15.61 |
| 1-7 d | 165 | 18.01 | 50 | 5.46 | 215 | 23.47 |
| 7-14 d | 17 | 1.86 | 4 | 0.44 | 21 | 2.29 |
| >14 d | 7 | 0.76 | 4 | 0.44 | 11 | 1.20 |
| Total | 673 | 73.47 | 346 | 26.53 | 916 | 100.00 |
Table 5: Distribution of ADE occurrence time
| Organ or system involved | Number of manifestations |
% | Main clinical manifestations |
|---|---|---|---|
| Skin and appendage disorders | 324 | 29.19 | Erythema multiforme, pruritus, rash, rash erythematous, rash maculopapular, skin striae, urticarial,flushing |
| Nervous system disorders | 112 | 10.09 | Agitation, anaesthesia local, confusion, somnolence, dizziness, headache, coma, tetany, vertigo, vocal cord paralysis |
| Gastrointestinal system disorders | 331 | 29.82 | Abdominal pain, appetite disorder, mouth dry, anorexia, constipation, diarrhoea, flatulence,increased stool frequency, nausea, vomiting, stomach discomfort |
| Body as a whole (general disorders) | 152 | 13.69 | Convulsions, anasarca, chills, body pain, muscle pain, fever, anaphylactic shock, anaphylactoid reaction, night sweats, periorbital oedema, peripheral oedema, oedema, pale, trembling, chest tightness, bone pain, fatigue, facial edema |
| Cardiovascular system disorders | 144 | 12.97 | Palpitation, hypotension, hypertension, cyanosis, phlebitis, tachycardia |
| Respiratory system disorders | 39 | 3.51 | Cough, dry cough, dyspnoea, asthma, larynx oedema |
| Metabolic and nutritional disorders | 4 | 0.36 | Hyperuricaemia, hypoproteinaemia, hypocalcaemia, hypokalaemia, |
| Urinary system disorders | 1 | 0.09 | Renal function abnormal |
| Psychiatric disorders | 1 | 0.09 | Mental disorder |
| Application site disorders | 2 | 0.18 | Injection site pain, injection site pruritus |
| Total | 1110 | 100.00 |
Table 6: Organs or systems involved in ADEs and clinical manifestations
The nature and causality evaluation of ADEs are shown in Table 7. Among the 916 ADEs, 733 (80.02%) were general, mainly involving the skin and its appendages, central and peripheral nervous systems, cardiovascular system and gastrointestinal system, and 24 (2.62%) were serious, mainly including allergy (especially anaphylactic shock), fainting, erythema, dyspnea, hyperpyrexia, and chest tightness.
| Nature | Number of cases | % | Causality evaluation | Number of cases | % |
|---|---|---|---|---|---|
| General | 733 | 80.02 | Definite | 251 | 27.40 |
| New | 159 | 17.36 | Probable | 344 | 37.55 |
| Serious | 24 | 2.62 | Possible | 271 | 29.59 |
| Possible irrelevant | 25 | 2.73 | |||
| To be evaluated | 20 | 2.18 | |||
| Unevaluated | 5 | 0.55 | |||
| Total | 916 | 100.00 | Total | 916 | 100.00 |
Table 7: Nature and causality evaluation of ADEs
Serious ADEs were concerned with 8 drugs, i.e. Yinzhihuang (6 cases), Kuhuang (1 case), hepatocyte growth-promoting factors (6 cases), diammonium glycyrrhizinate (2 cases), ribonucleic acid (2 cases), polyene phosphatidylcholine (3 cases), magnesium isoglycyrrhizinate (3 cases) and ademetionine (1 case). No rare ADEs or deaths occurred.
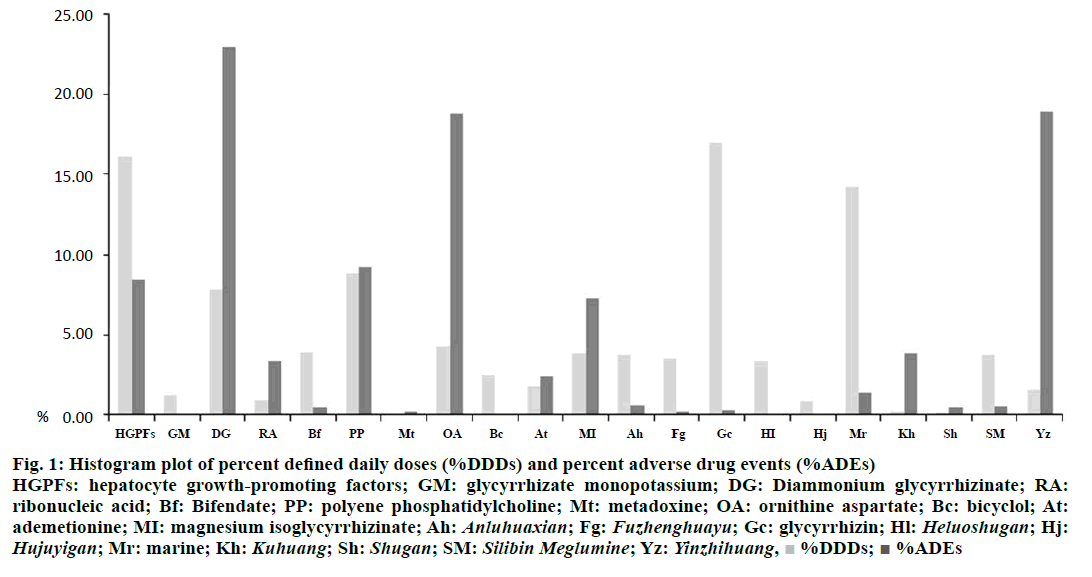
The relationship between %DDDs and %ADEs was plotted by histogram (Figure 1). Glycyrrhizin was used most frequently among TCMs, which accounted for 16.94% of total utilization of hepatoprotectives, while its percentage of ADEs was only 0.33%. In addition, Heluoshugan was also widely used (3.36%) and its percentage of ADEs was only 0.11%. In contrast, Yinzhihuang was not used as frequently as the above TCMs, whereas it led to higher percentage of ADEs (18.89%). Among chemical drugs, bifendate and bicyclol were used with higher percentages (3.93% and 2.54%, respectively), and with lower percentages of ADEs (0.44% and 0.11%, respectively). On the contrary, ribonucleic acid was not used as frequently as the above chemical drugs, whereas they lead to higher percentage of ADEs (3.38%). It was noteworthy that ornithine aspartate and diammonium glycyrrhizinate were used with high percentages (4.35% and 7.82%, respectively), and led to higher percentages of ADEs (18.78% and 22.93%, respectively). Therefore, safety of certain hepatoprotectives should be taken into consideration in clinical therapy.
Figure 1: Histogram plot of percent defined daily doses (%DDDs) and percent adverse drug events (%ADEs)
HGPFs: hepatocyte growth-promoting factors; GM: glycyrrhizate monopotassium; DG: Diammonium glycyrrhizinate; RA:
ribonucleic acid; Bf: Bifendate; PP: polyene phosphatidylcholine; Mt: metadoxine; OA: ornithine aspartate; Bc: bicyclol; At:
ademetionine; MI: magnesium isoglycyrrhizinate; Ah: Anluhuaxian; Fg: Fuzhenghuayu; Gc: glycyrrhizin; Hl: Heluoshugan; Hj: Hujuyigan; Mr: marine; Kh: Kuhuang; Sh: Shugan; SM: Silibin Meglumine; Yz: Yinzhihuang,  %DDDs;
%DDDs;  %ADEs
%ADEs
Data on drug utilization and ADR report system are best viewed as the central components of a comprehensive post-marketing surveillance program [13-16]. In China, a regional hospital drug information system in which six provinces took part, Yangtze River hospital drug information network, was established in 1999. Drug utilization data are submitted to the network annually and can be monitored or analysed dynamically.
Different countries use different ways to monitor ADR. In the USA, voluntary and mandatory reporting systems co-exist [17]. Unlike that of USA, Chinese ADR monitoring system adopts a decentralized management model with 34 regional centers as its main component [18], and hospital-based monitoring is one of the major methods used to collect ADR. The data of drug utilization and ADEs in Hubei Province are all obtained from the above two databases.
Of the 916 ADEs caused by hepatoprotectives, the most commonly affected age group was 41 to 70 y (65.83%) (Table 4). Males were more vulnerable than females. Due to the decline in kidney function and combination therapy, the elderly tolerate these drugs poorly [19]. Thus, it is crucial for doctors to take the special physiological conditions and medication contraindications of older people into consideration to prevent adverse reactions [20]. However, it remained unknown whether people aged 41 to 70 y were really prone to ADEs or whether they were simply the most likely group to be treated with hepatoprotective drugs.
The study also revealed that most of the ADEs (87.62%) and all of the serious ADEs were caused by intravenous hepatoprotectives, probably because intravenous infusion was not only quicker than oral administration, but also directly into the blood without the liver first-pass effect, thus inducing ADEs more easily. Therefore, effective oral administration of hepatoprotectives is better than intravenous infusion unless there is an emergency or a dangerous condition. Furthermore, most ADEs occurred in the first 30 min of the first administration, suggesting that patients should be closely observed in the early period after infusion. Meanwhile, close attention should be paid to the patients receiving continuous hepatoprotective treatment.
Irrational use of hepatoprotectives, such as off-label use, irrational drug combination, overuse and inappropriate menstruum, may also lead to ADEs. For example, treating whole blood cell reduction with Yinzhihuang was off-label use. As indicated by the instructions of Kuhuang, the best dose on the first day is 10 ml, while the patient was overdosed through intravenous infusion (50 ml), which caused a serious ADE. Therefore, in order to reduce the incidence of ADEs, hepatoprotectives should be used strictly according to the instructions that are as simple as possible, and can be combined based on the best evidence if necessary.
Provided that hepatoprotectives have similar safety, ADEs occur more frequently with increasing frequency of the agent used. However, in this study, 11/21 (52.38%) hepatoprotectives were chemical drugs, which accounted for 51.64% of total DID, but 78.95% of total expenditure and 73.47% of all ADEs (Table 2 and 4). In general, TCMs were seemingly but not invariably safer and cheaper than chemical drugs [21,22]. Glycyrrhizin, as one of the oral TCMs, was widely used with 606.25 DID, only causing 3 ADEs, which indicated its safety in clinical therapy. A Medline search also showed that this agent barely had adverse effects. In contrast, ribonucleic acid was one of the injectable chemical drugs, which was used with 34.79 DID but caused 31 ADEs. Yinzhihuang was used with 57.31 DID, whereas it led to 173 ADEs, with 6 cases being serious. Thus, the Chinese regulatory authority has also issued several regulations to minimize the risks of ADEs for TCM [23-26].
Notably, there are some limitations to this study. First, our results may underestimate hepatoprotective utilization in children or those with renal failure, for DDDs are based on average doses for adults. Second, ADEs may be under-reported due to hospital-based monitoring ADEs system in China, for the public may have problems recognizing the scenario as an ADE and lack recognition of the importance of ADE reporting [27,28]. Third, only 251 (27.40%) ADEs were evaluated as definite causality with the agents (Table 7). Rechallenge does not often occur in clinical practice, so the causality between ADE and drugs can hardly be determined. Last, the large databases should be added and this study may not be generalized to the other culture.
Our combined, the analysis revealed that the consumption sum and DID of hepatoprotectives kept increasing from 2012 to 2014, and caused relatively high incidences of ADEs. Irrational use and intravenous administration were associated with an increased risk of ADEs, therefore, surveillance and educational strategies should be strengthened successfully to promote the safety of hepatoprotective drugs. Of the 21 hepatoprotectives, Glycyrrhizin, Heluoshugan, and Bicyclol were preferable for clinical hepatoprotection, whereas Yinzhihuang, Kuhuang, and ribonucleic acid should be avoided. This parallel approach through spontaneous reporting and drug utilization analyses provided valuable information for the safety of hepatoprotectives, and this synergy should be encouraged to support future pharmacovigilance activities.
Acknowledgements
The authors acknowledge the important contribution of the Yangtze River Hospital Drug Information Centre and HAERS participants in providing data.
Conflict interests
The authors declare that there is no conflict of interest.
Financial support and sponsorship
Nil.
References
- Chinese Society of Hepatology and Chinese Society of Infectious Diseases, Chinese Medical Association. The guideline of prevention and treatment for chronic hepatitis B (2010 version). Zhonghua Gan Zang Bing Za Zhi 2011;19:13-24.
- Expert Committee of Liver Inflammation and its Prevention. Expert Consensus on the Prevention and Treatment of Hepatic Inflammation and Injury. Chin J Pract Intern Med 2014;34:152-62.
- Joo SS, Kang HC, Won TJ, Lee DI. Ursodeoxycholic acid inhibits pro-inflammatory repertoires, IL-1 beta and nitric oxide in rat microglia. Arch Pharm Res 2003;26:1067-73.
- Feher J, Lengyel G. Silymarin in the treatment of chronic liver diseases: past and future. Orv Hetil 2008;149:2413-8.
- Ram VJ, Goel A. Past and present scenario of hepatoprotectants. Curr Med Chem 1999;6:217-54.
- Long LH, Xue CQ, Shi JF, Dong JN, Wang L. Efficacy of hepatoprotective agents with or without antiviral drugs on liver function and fibrosis in patients with hepatitis B: a meta-analysis. Hepat Mon 2015;15:e29052.
- Ram VJ, Goel A. Present status of hepatoprotectants. Prog Drug Res 1999;52:53-101.
- Gong L. Clinical rational use of drugs for the treatment of liver and gallbladder diseases. Evaluation and Analysis of Drug-use in Hospitals of China 2013;13:103-5.
- http://www.whocc.no/atc_ddd_index/.
- http://www.sda.gov.cn/WS01/CL0053/62621_7.html.
- Ren X, Liu D, Ding N, Huang K, Xiong Y, Du G, et al. Safety evaluation of cephalosporins based on utilization and adverse drug events: analysis of two databases in China. Expert Opin Drug Saf 2012;11:689-97.
- http://www.xjda.gov.cn/WS01/CL0290/15737.html.
- Raschi E, Poluzzi E, Godman B, Koci A, Moretti U, Kalaba M, et al. Torsadogenic risk of antipsychotics: combining adverse event reports with drug utilization data across Europe. PloS One 2013;8:e81208.
- Poluzzi E, Raschi E, Godman B, Koci A, Moretti U, Kalaba M, et al. Pro-arrhythmic potential of oral antihistamines (H1): combining adverse eventreports with drug utilization data across Europe. PloS One 2015;10:e0119551.
- Rodriguez EM, Staffa JA, Graham DJ. The role of databases in drug postmarketing surveillance. Pharmacoepidemiol Drug Saf 2001;10:407-10.
- Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol 2005;58:323-37.
- Trontell AE. How the US Food and Drug Administration defines and detects adverse drug events. Curr Ther Res Clin Exp 2001;62:641-9.
- Montastruc JL, Sommet A, Lacroix I, Olivier P, Durrieu G, Damase-Michel C, et al. Pharmacovigilance for evaluating adverse drug reactions: value, organization, and methods. Joint Bone Spine 2006;73:629-32.
- Fan ZH. Analysis of ADR in old patients. Guide of China Medicine 2008;6:127-8.
- Huang JH, Liang Q. Enhancement of drug monitoring to prevent ADR. J Pract Med Tech 2005;12:3641-2.
- Wang L, Yuan Q, Marshall G, Cui X, Cheng L, Li Y, et al. Adverse drug reactions and adverse events of 33 varieties of traditional Chinese medicine injections on National Essential medicines List (2004 edition) of China: an overview on published literatures. J Evid Based Med 2010;3:95-104.
- Gao P, Zhang CL, Zeng FD, Pu R, Zhang J, Huang R, et al. Pharmacovigilance in China: issues of concern identified through an analysis of the Chinese Adverse Drug Reaction Information Bulletin 2001 to 2014. J Clin Pharm Ther 2015;40:594-8.
- http://www.sda.gov.cn/WS01/CL0058/15768.html/.
- http://www.sda.gov.cn/WS01/CL0053/24466.html/.
- http://www.sda.gov.cn/WS01/ CL0055/10637.html/.
- http://www.sda.gov.cn/WS01/CL0055/27058.html/.
- Xia J, Zhang X. Discussion on the influential factors of low reported rate in spontaneous reporting system. J Pharma Epidemiol 2012;21:35-7.
- Gauld NJ, Shaw JP, Emmerton LM, Pethica BD. Surveillance of a recently switched non-prescription medicine (Diclofenac) using a pharmacy-based approach. Pharmacoepidemiol Drug Saf 2000;9:207-14.