- *Corresponding Author:
- G. Szakonyi
Institute of Pharmaceutical Analysis, Faculty of Pharmacy, University of Szeged, H-6720 Szeged, Somogyi u. 4., Hungary
E-mail: gerda.szakonyi@pharm.u-szeged.hu
| Date of Submission | 22 April 2013 |
| Date of Revision | 24 November 2013 |
| Date of Acceptance | 1 December 2013 |
| Indian J Pharm Sci 2014;76(1):31-37 |
Abstract
Suppositories are important tools for individual therapy, especially in paediatrics, and an instrumental assay method has become necessary for the quality control of dosage units. The aim of this work was to develop a rapid, effective high-performance liquid chromatography method to assay aminophenazone in extemporaneous suppositories prepared with two different suppository bases, adeps solidus and massa macrogoli. With a novel sample preparation method developed by the authors, 4-dimethylaminoantipyrine was determined in these suppository bases with 95-105% recovery. The measurements were carried out on a Shimadzu Prominence ultra high-performance liquid chromatography system equipped with a 20 Î…l sample loop. The separation was achieved on a Hypersil ODS column, with methanol, sodium acetate buffer (pH 5.5±0.05, 0.05 M, 60:40, v/v) as the mobile phase at a flow rate of 1.5 ml/min. The chromatograms were acquired at 253 nm. The chromatographic method was fully validated in accordance with current guidelines. The presented data demonstrate the successful development of a rapid, efficient and robust sample preparation and high-performance liquid chromatography method for the routine quality control of the dosage units of suppositories containing 4-dimethylaminoantipyrine.
Keywords
HPLC, validation studies, 4-dimethylantipyrine, suppositories, analytic sample preparation
Suppositories are currently very popular formulations especially in paediatrics, where they can be used for the effective lowering of fever. The choice of a suppository as the mode of drug delivery is justified in all cases when oral delivery is impossible, that is an unconscious or vomiting patient, or in the case of infants. The therapeutic effect of correctly applied suppositories can be compared with that of injections because the active substance can penetrate from the lower tract of the rectum to the inferior vena cava, thereby avoiding the vena portae and the liver, and can exert a systemic effect when transported to the vena cava inferior. Currently suppositories are actuality used therapeutically all over the world. Suppository is being widely used for several indications utilising its feature that local effect of the suppository can be transformed into therapeutic benefit (e.g. in case of treatment of ulcerative colitis, ulcerative proctitis or colorectal cancer in paediatric practise) [1-3]. Treatment of acute malaria in case of children requires combination therapy in order to avoid development of multidrug resistance. In these scenarios it is a plausible solution to deliver one of the drugs of the combination in suppository [4,5]. Thus rapid systemic effect can be achieved. For the delivery of several nonsteroid antiinflammatory drugs, such as paracetamol or indomethacin, efficacy of suppository form is equivalent with or superior to oral route [6,7]. Therefore, the quality control of this widely applied dosage form with a modern, instrumental analytical method is highly desired.
In Hungarian pharmaceutical practice, extemporaneous products including suppositories are just as popular as factory-produced medicines. Extemporaneous products comprise part of personal therapy, and take into account the physical status, age and other diseases of the patient. Extemporaneously produced pharmaceuticals are used particularly in paediatric clinical departments, where they are the most effective means of lowering fever.
4-Dimethylaminoantipyrine (aminophenazone, AMFZ) is an antipyretic and analgesic that is frequently used in paediatric practice in Hungary. This molecule is also referenced in literature as 4-dimethylamino-1,5-dimethyl-2-phenyl-1,2- dihydro-3H-pyrazol-3-on, aminopyrin, pyramidon, dimethylaminophenyldimethylpyrazolon, 2,3-dimethyl- 4-dimethylamino-1-phenyl-5-pyrazolon or dimethylaminophenazon.
Numerous manufacturers market antipyretic suppositories in various dosage strengths for neonates and infants. The antifebrile effect of AMFZ develops especially quickly (comparable to that of injections) if the drug is taken rectally. An additional benefit is that its administration does not require specially trained staff.
Agranulocytosis, one of the registered sideeffects of the substance, has a very low incidence, while carcinogenicity, another possible side-effect, can be completely eliminated through rectal administration [8-16]. During its biotransformation [8-16], AMFZ is demethylated [13,16,17] in two steps, catalysed by cytochrome P450 2B [9,10]. The demethylated product then undergoes acetylation [12] and is eliminated from the body as acetylaminoantipyrine. In the presence of nitrite ion at pH between 2.0 and 3.1, the carcinogenic nitrosamine derivative dimethylnitrosamine is formed [8] in parallel with the demethylation. The physiological circumstances in the stomach provide a suitable medium for this reaction to take place [17-19]. In contrast, the rectal administration of AMFZ completely eliminates the possibility of dimethylnitrosamine formation as the pH of the mucous fluid in that region is around 7.9.
In consequence of the increased application of suppositories, there is a current demand for a rapid, effective and state-of-the-art reversed-phase chromatographic method for routine analysis. Our literature search revealed that methods for the high-performance liquid chromatography (HPLC) analysis of AMFZ are very rare and those found related to very low concentrations in biological fluids or tissues [20-23]. Our aim was therefore to develop and validate a suitable general sample preparation and chromatographic method for suppositories containing AMFZ. Moreover, HPLC analysis of the pyrazolone derivative metamizole in tablet formulations could shed light on the initial steps of method development [23-27].
Materials and Methods
Throughout this investigation, HPLC grade organic solvents were used. Methanol was obtained from Merck (LiChrosolv, Darmstadt, Germany) and Sigma- Aldrich (Chromasolv for HPLC, St. Louis, MO, USA). The buffer solutions were prepared by using triple distilled water. The pH of the buffer solutions was set to the desired value by using sodium acetate anhydrate (Reanal) and acetic acid 96% (Molar Chemicals). 4-Dimethylaminoantipyrine (Sigma- Aldrich) was used as reference substance.
Suppositories based on adeps solidus (hard fat; Ph. Eur. 7.4) or on massa macrogoli (a hydrophilic suppository base sorbitan monolaurate and macrogol 1540 in 95:5 w/w ratio), without or with the active substance (100 mg of AMFZ), were utilised for the accuracy and specificity studies of the validation and during the method development; they were provided by the Pharmacy of the University of Szeged.
Repeatability and intermediate precision studies were carried out with Suppositorium antipyreticum pro parvulo prepared according to Formulae Normales VII. (a collection of standard prescriptions in Hungary), produced by Naturland Hungary Ltd. (Budapest, Hungary), in order to ensure the best homogeneity of the samples.
Paediatric suppositories that contain 100 mg of AMFZ exclusively are not marketed by pharmaceutical companies. These reference suppositories for the repeatability and intermediate precision measurements were therefore provided by the Institute of Pharmaceutical Technology of the Faculty of Pharmacy, University of Szeged, prepared as prescribed by the authors: 12 suppositories were prepared containing 100 mg of AMFZ in each dosage unit. All dosage units used during the method development and validation were prepared through the use of moulds for infant suppositories.
Instruments and conditions
All measurements were carried out using a Shimadzu Prominence (Shimadzu Corp., Japan) ultra high-performance liquid chromatography (UHPLC) system, consisting of an LC-20AD high-pressure pump equipped with a 4-way solenoid mixing valve, a CT0-20A column thermostat and an SPD-M20A UV/Vis Diode Array detector equipped with a 10 mm optical path length flow cell. The injections were carried out via a Rheodyne manual injection valve fitted with a 20 μl sample loop. The separation was achieved on a Hypersil ODS (C18) 150×4.6 mm, 5 μm column (Thermo Scientific, Keystone, UK). The flow rate of the mobile phase was 1.5 ml/min. The composition of the mobile phase was selected on the basis of the results obtained from Pallas chromatographic prediction software [21]. The mobile phase was methanol–sodium acetate buffer (pH 5.5; 0.05 M) (60:40, v/v). The chromatograms were acquired at 253 nm for 5 min. The chromatograms were integrated by means of LCSolution software (Shimadzu Corp.)
Chromatographic method development
The stationary phase was chosen on the basis of the work of El-Seikh et al. [20], but our initial experiments revealed that the composition and the pH of the mobile phase had to be changed considerably. With methanol–acetic acid (pH 2.78; 1.0%) (70:30, v/v) as mobile phase, the AMFZ peak eluted between 15 and 30 min and showed significant asymmetry. It was obvious that the mobile phase composition described by El-Seikh et al. would have given a much longer retention time. Simulations carried out with the Pallas software [28] showed that the pH should be >4.5 to achieve acceptable robustness and peak shape. A set of experiments was therefore designed using methanol–sodium acetate buffer (pH 4.5 or 5.0; 0.05 M, 50:50 or 60:40, v/v) as mobile phase in the various combinations. The shape of the AMFZ peak in the resulting chromatograms improved on increase of both the pH and the proportion of methanol. In the final experiment, with methanol–sodium acetate buffer (pH 5.5; 0.05 M) (60:40, v/v) as eluent, the symmetry factor of the AMFZ peak proved to be 1.43, and the peak width measured at the baseline was 0.2 min. It still seemed plausible to use acetate buffer at pH 5.5, where it has a somewhat lower buffer capacity, but the chosen concentration of 0.05 M compensates this.
Preparation of samples for analysis
Extemporaneous prescriptions do not usually specify the suppository base to be used as vehicle and it is left to the pharmacist to apply his or her professional knowledge to choose the most suitable one from the possibilities listed in the official Pharmacopoeia. The development of the sample preparation involved in particular two suppository vehicles, adeps solidus and massa macrogoli, as these are the most commonly chosen ones. The same methanol–water solvent mixture (50:50, v/v) was used for both vehicles. However, the methods differed as concerns other aspects of the sample preparation. This is due to the fundamentally different physico-chemical properties of these two vehicles.
Adeps solidus and massa macrogoli cannot be distinguished by purely organoleptic examination. In the first step of sample preparation, the suppository (containing the unidentified vehicle) was weighed in a beaker, 15 ml of the above solvent mixture was added, and the beaker was heated in a 40° water bath until the suppository melted. (At this point, the behaviour of the molten suppository reveals its nature). In the case of adeps solidus, a consistent, clear, colourless fatty phase appears on the surface of the solvent mixture, whereas with massa macrogoli the solution becomes homogeneous and clear and no second phase can be observed. In some cases, massa macrogoli may contain a certain amount of tensides, when the resulting solution is opaque, but even then no second phase or fat droplets can be observed.
At this stage, the active substance was extracted from the vehicle by shaking the sample for 10 min.
The massa macrogoli-based samples did not require filtration, so the solution was transferred directly into a 50 ml volumetric flask and the beaker was washed out with another 15 ml and then 2×5 ml of solvent mixture, the washings likewise being transferred to the volumetric flask, the solution next being made up to volume with the solvent mixture.
The adeps solidus-based samples required removal of the fatty phase by freezing on an ice-bath, when the fat solidified and the liquid could be decanted into a 50 ml volumetric flask. This extraction step was repeated with a second 15 ml portion of solvent mixture in a 40° water bath. The beaker was finally washed twice with 5 ml of solvent mixture, which was transferred to the volumetric flask, the solution then being made up to volume with the solvent mixture. The outstanding benefit of this sample preparation procedure is that it does not require an initial knowledge of the suppository base used.
Finally, in both cases a 0.3 ml aliquot of the stock solution was transferred to a 10 ml volumetric flask and made up to volume with the solvent mixture. The solution was filtered on a Millipore Millex PVDF membrane filter with a pore size of 0.45 μm.
Preparation of standard solution and establishment of system suitability
The AMFZ contents of the samples were quantified by reference to a standard AMFZ solution with a concentration of 0.075 mg/ml in the same solvent mixture, which corresponded to the theoretical 100% concentration level of the sample solutions to be examined. Two standard solutions were prepared from independent stock solutions in order to check the system suitability by the following procedure.
The precision of the injections was checked before all measurement sets by injecting the first standard solution five times. The system was considered suitable if the relative standard deviation percent (RSD%) of the five replicate injections did not exceed 2.0%. The accuracy of the calibration was checked by injecting the second standard solution twice. The results were accepted if the correlation factor calculated from the average response ratio of the two standard solutions did not exceed 2.0.
Correlation factor was calculated by the following formula, [1-(AStd1×wStd2)/(AStd2×wStd1)]×100%; where AStd1 and AStd2 are the average peak areas of the replicate standard injections, while wStd1 and wStd2 are the weights of the standard substances used to prepare the solutions. The symmetry factor of the main peak of interest was also monitored throughout the measurements; it had to be between 0.7 and 2.0 for the analysis to be started.
Validation
A full validation of the method according to International Conference on Harmonisation (ICH) guideline Q2 (R1) [29], including linearity, repeatability, intermediate precision, accuracy, specificity and robustness have been performed. As the method was to be utilised for the rapid quality control of dosage units, which does not require the method to be stability-indicating, forced degradation studies were not conducted [30]. The repeatability, intermediate precision, accuracy and specificity studies were carried out with both vehicles.
Linearity:
The linearity of the method was examined in the concentration range between 0.025 and 0.150 mg/ml, which corresponds to 50–450% of the nominal content of the suppositories. The higher limit was chosen with regard to the fact that initial experiments gave individual results in this concentration range. Thus, it was necessary to check the method at extremely high active substance concentrations. The range was covered by seven solutions each diluted from two individually prepared reference solutions so that the sequence of the stock solutions used for the dilutions alternated. The peak areas determined with LCSolution were plotted versus the concentration of the solutions and a straight line was fitted to the points. The slope of the fitted straight line was found to be 3.498 107, the intercept was -5.165 104 and R2 was 0.9998. This proved that in the proposed concentration range the method was linear.
Precision and repeatability
Repeatability was checked on six individual suppositories prepared according to the method described in chromatographic method development section. In the case of adeps solidus as vehicle, one of the six replicate results exceeded the 125% limit, and this result was omitted from the calculation of the RSD%. On the basis of our result that there is no carryover between the injections and the fact that active substance was not added to the solution. RSD% proved to be 1.4%, which can be considered acceptable when it is taken into account that each sample preparation was made from different individual suppositories and not from a composite sample of multiple suppositories. The massa macrogoli-based suppositories gave an RSD% of 2.1%, which is also acceptable.
Intermediate precision
The same analytical procedure was carried out by another analyst on another day, using a freshly prepared mobile phase. Relative differences between average results of 2 days were calculated with the following formula: (X̅ Day1-X̅ Day2)×2/(X̅ Day1- X̅ Day2)×100%, where X̅ Day1 denotes the average result of the specific day. The results for the adeps solidus-based samples were an RSD% of 1.2% and a relative difference of 1.3% between the averages of the repeatability (Day 1) and intermediate precision (Day 2) results compared to the mean of the average values measured for each. Both results can be accepted according to the principles of general pharmaceutical analytical practice. For the massa macrogoli-based samples, the RSD% of the individual results was 2.5%, while the relative difference between the repeatability and intermediate precision was 3.7%. Both results are in accordance with the appropriate guidelines, and are therefore considered acceptable.
Accuracy
The accuracy of the method was studied between 50% and 450% of the nominal content of the suppositories, that is, 100 mg. The results are shown in Table 1. Although all of the average values fell between 95% and 105%, it should be mentioned that in the case of adeps solidus most of the averages were below 100%, while in the case of massa macrogoli they were above 100%. This may raise a warning flag, but there was no trend within the results that could be correlated with the increasing concentration of the sample groups.
| Level % | Adeps solidus | Massa macrogoli | ||||||
|---|---|---|---|---|---|---|---|---|
| Replicates % Mean % RSD% Replicates % Mean % RSD% | ||||||||
| 50 | 1. | 98.7 | 99.4 | 0.63 | 1. | 104.10 | 102.5 | 1.56 |
| 2. | 99.9 | 2. | 100.90 | |||||
| 3. | 99.6 | 3. | 102.40 | |||||
| 100 | 1. | 99.5 | 100.3 | 0.75 | 1. | 97.40 | 99.7 | 1.99 |
| 2. | 100.3 | 2. | 100.50 | |||||
| 3. | 101.0 | 3. | 101.10 | |||||
| 150 | 1. | 96.0 | 95.3 | 1.36 | 1. | 104.60 | 104.9 | 0.42 |
| 2. | 93.8 | 2. | 105.40 | |||||
| 3. | 96.1 | 3. | 104.70 | |||||
| 300 | 1. | 96.2 | 95.5 | 0.87 | 1. | 104.60 | 102.7 | 2.43 |
| 2. | 95.8 | 2. | 103.70 | |||||
| 3. | 94.6 | 3. | 99.90 | |||||
| 450 | 1. | 96.3 | 96.5 | 0.55 | 1. | 102.80 | 102.5 | 1.49 |
| 2. | 96.1 | 2. | 103.80 | |||||
| 3. | 97.1 | 3. | 100.80 | |||||
Table 1: Results Of The Accuracy Studies
Stability of standard and sample solutions
Stability of the standard solution and the sample solution was studied for 4 days. Both solutions were stored in a refrigerator between 2° and 8°. The acceptance criterion was set up according to the relative difference value defined by the following formula: [(AStart-AStored)/AStart]×100%. The solution was considered stable as long as the relative difference at a specific time point was lower than 3.0%. On the basis of the data presented in Table 2, the standard solutions can be considered stable for at least 96 h, and the sample solutions can be considered stable for at least 96 h.
| Time (h) | Standard solution | Sample solution | ||
|---|---|---|---|---|
| Area | Relative difference (%) | Area | Relative difference (%) | |
| 0 | 2 903 843 | ‑ | 2 076 275 | ‑ |
| 18 | 2 909 154 | 0.2 | 2 075 720 | 0.0 |
| 24 | 2 904 895 | 0.0 | 2 073 721 | ‑0.1 |
| 39 | 2 902 092 | ‑0.1 | 2 074 466 | ‑0.1 |
| 48 | 2 904 450 | 0.0 | 2 073 840 | ‑0.1 |
| 63 | 2 906 272 | 0.1 | 2 074 709 | ‑0.1 |
| 72 | 2 903 753 | 0.0 | 2 080 587 | 0.2 |
| 96 | 2 904 386 | 0.0 | 2 077 305 | 0.0 |
Table 2: Results Of The Solution Stability Studies
Specificity
When the procedure was carried out with blank suppositories (containing no active substance), no peak was detected at the retention time of AMFZ. It can be stated that there are so excipients in either vehicles that interfere with the determination of AMFZ.
Robustness
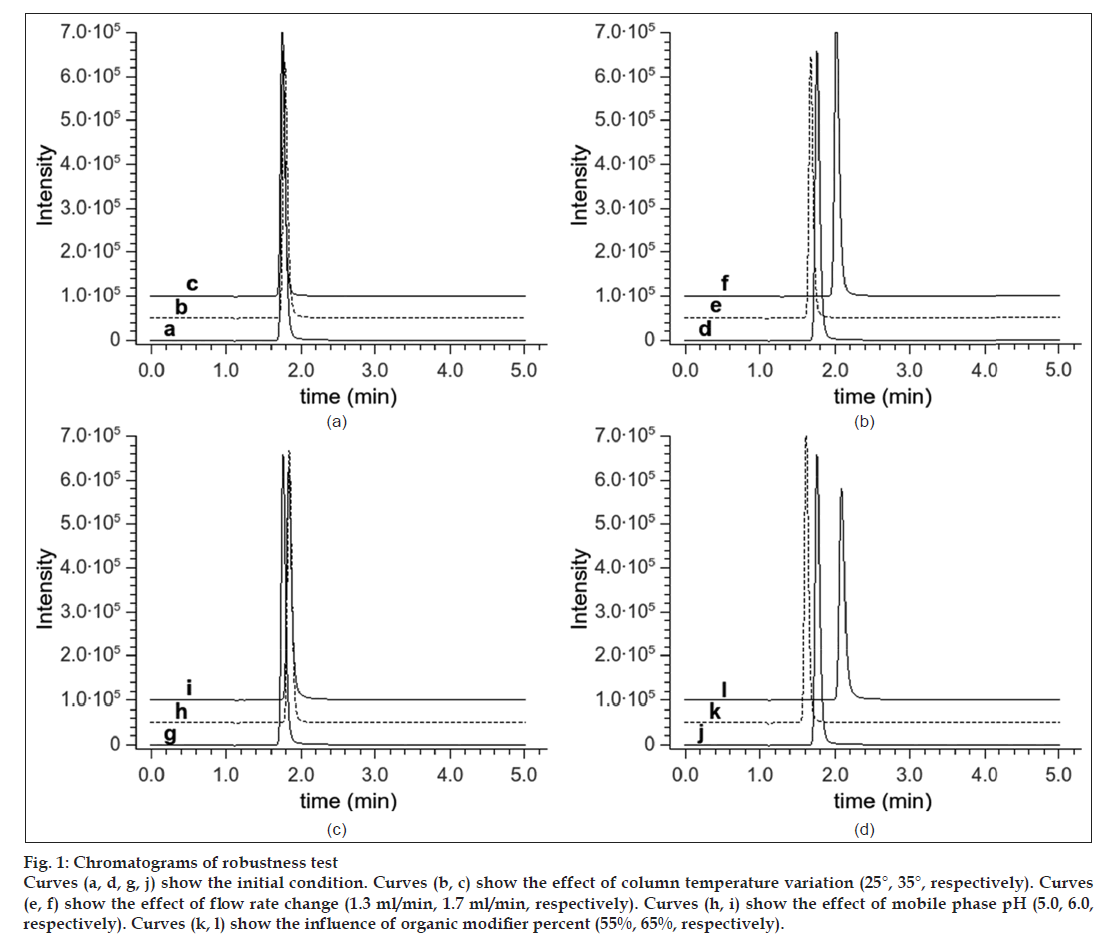
The effects of changing the organic–aqueous ratio, the pH of the aqueous phase, the flow rate of the mobile phase and the temperature of the column on the retention time and on the shape of the AMFZ peak were examined. The results of the robustness study presented in Table 3 demonstrate that the ratio of the aqueous and organic phases exerted a great influence on both the retention time and the peak symmetry of the analyte. The pH of the aqueous phase significantly changed the symmetry of the peak, which is in accordance with the results obtained from the simulations with the Pallas software. The lower the pH, the more asymmetrical the peak was. In contrast, the pH of the mobile phase had only a very slight effect on the retention time of the peak. The flow rate influenced the retention time, as expected, while it had a negligible effect on the peak shape. The column temperature did not influence either the retention time or the symmetry of the main peak. The acquired chromatograms can be seen in fig. 1.
| Condition changed (Units) | tR (min) | N | Symmetry factor |
|---|---|---|---|
| Aqueous: Organic ratio | |||
| 45:55 | 2.088 | 3973 | 1.535 |
| 40:60 | 1.761 | 4074 | 1.434 |
| 35:65 | 1.616 | 4512 | 1.460 |
| Buffer pH | |||
| 5.00 ± 0.05 | 1.837 | 3747 | 1.602 |
| 5.50 ± 0.05 | 1.761 | 4074 | 1.434 |
| 6.00 ± 0.05 | 1.846 | 4441 | 1.346 |
| Flow rate (ml/min) | |||
| 1.3 | 2.021 | 4346 | 1.432 |
| 1.5 | 1.761 | 4047 | 1.434 |
| 1.7 | 1.676 | 4117 | 1.405 |
| Column temperature (°C) | |||
| 25 | 1.785 | 3890 | 1.433 |
| 30 | 1.761 | 4074 | 1.434 |
| 35 | 1.751 | 4340 | 1.408 |
Table 3: Results Of The Robustness Studies
Results and Discussion
On the basis of the results presented here the proposed method is appropriate for the determination of AMFZ in the concentration range 0.025-0.150 mg/ ml with excellent repeatability, intermediate precision and accuracy. The chromatographic method is robust with respect to changing the parameters between the boundaries presented in Table 3. Retention parameter changes of the AMFZ peak are in excellent agreement with the expected behaviour in case of changing the flow rate, column temperature or ratio of the organic modifier. Changing the buffer pH did not influence the retention time or number of theoretical plates of AMFZ but had significant effect on the peak shape of the component.
The data presented in this paper reveal that a rapid, efficient and robust sample preparation procedure and HPLC method were successfully developed and fully validated for the routine quality control of the dosage units of suppositories containing AMFZ as active substance in various vehicles as supporting materials. The method is simple and sufficiently general to be conveniently used for the regular quality control of AMFZ suppositories formulated through the use of different suppository bases.
Acknowledgements
This work was supported by grants, TÁMOP-4.2.1/B-09/1/ KONV-2010-0005, TÁMOP-4.2.2/B-10/1-2010-0012 and the European Regional Development Fund through grant ERC _HU_09 3D_TRPV1. The authors are also grateful to the Pharmacy of the University of Szeged and the Institute of Pharmaceutical Technology, Faculty of Pharmacy, University of Szeged, for providing the suppository samples. The authors express their thanks to the Analytical Development Department of the Generic R and D Division of Teva Pharmaceuticals Ltd, Hungary, for permission to use the Pallas chromatographic prediction software.
References
- Okabayashi K, Hasegawa H, Watanabe M, Ohishi T, Hisa A, Kitagawa Y. Usefulness of the preoperative administration of tegafur suppositories as alternative adjuvant chemotherapy for patients with resectable stage II or III colorectal cancer: A KODK4 multicenter randomized control trial. Oncology 2012;83:16-23.
- Richter JM, Kushkuley S, Barrett JA, Oster G. Treatment of new-onset ulcerative colitis and ulcerative proctitis: a retrospective study. Aliment Pharmacol Ther 2012;36:248-56.
- Heyman M, Kierkus J, Spenard J, Shbaklo H, Giguere M. Efficacy and safety of mesalamine suppositories for treatment of ulcerative proctitis in children and adolescents. Inflamm Bowel Dis 2010;16:1931-9.
- Sabchareon A, Attanath P, Chanthavanich P, Phanuaksook P, Prarinyanupharb V, Poonpanich Y, et al. Comparative clinical trial of arthesunate suppositories and oral artesunate in combination with mefloquine in the the treatment of children with acute falciparum malaria. Am J Trop Med Hyg 1998;58:11-6.
- Cao XT, Bethell DB, Pham TP, Ta TT, Tran TN, Nguyen TT, et al. Comparison of artemisinin suppositories, intramuscular artesunate and intravenous quinine for the treatment of severe childhood malaria. Trans R Soc Trop Med Hyg 1997;91:335-42.
- Hadas D, Youngster I, Cohen A, Leibovitch E, Shavit I, Erez I, et al. Premarketing surveillance of ibuprofen suppositories in febrile children. Clin Pediatr 2012;50:196-9.
- Scolnik D, Kozer E, Jacobson S, Diamond S, Young NL. Comparison of oral versus normal and high-dose rectal acetaminophen in the treatment of febrile children. Pediatrics 2002;110:553-6.
- Mirvish SS, Gold B, Eagen M, Arnold S. Kinetics of the nitrosation of aminopyrine to give dimethylnitrosamine. Clin Oncol 1974;82:259-68.
- Roberts AG, Sjögren SE, Formina N, Vu KT, Almutairi A, Halpert JR. NMR-derived models of amidopyrine and its metabolites in complexes with rabbit cytochrome P450 2B4 reveal a structural mechanism of sequential N-dealkylation. Biochemistry 2011;50:2123-34.
- Agúndez JA, Martínez C, Benítez J. Metabolism of aminopyrine and derivatives in man: In vivo study of monomorphic and polymorphic metabolic pathways. Xenobiotica 1995;25:417-27.
- Gram TE, Wilson JT, Fouts JR. Some characteristics of hepatic microsomal systems which metabolize aminopyrine in the rat and rabbit. J Pharmacol Exp Ther 1968;159:172-81.
- Agúndez JA, Carillo JA, Martínez C, Benítez J. Aminopyrine Metabolism in Man: The Acetylation of AminoantipyrineCosegregates with Acetylation of Caffeine. Ther Drug Monit 1995;17:1-5.
- Noda A, Tsubone N, Mihara M, Goromaru T, Iguchi S. Formation of 4-formylaminoantipyrine as a new metabolite of aminopyrine. II. Enzymatic demethylation and oxidation of aminopyrine and 4-monomethylaminoantipyrine. Chem Pharm Bull 1976;24:3229-31.
- Hermann G. On aminophenazone metabolism in the isolated perfused rat liver. Eur J Drug Metab Pharmacokinet 1976;1:182-7.
- Levy M, Zylber-Katz E, Rosenkranz B. Clinical pharmacokinetics of dipyrone and its metabolites. Clin Pharmacokinet 1995;28:216-34.
- Bast A, Noordhoek J. Inhibition of aminopyrine demethylation and binding to cytochrome P-450 by its main metabolites in rat liver microsomes. Br J Pharmacol 1980;68:121-2.
- Sunderman FW Jr, Leibman KC. Nickel carbonyl inhibition of induction of aminopyrine demethylase activity in liver and lung. Cancer Res 1970;30:1645-50.
- Inai K, Kobuke T, Fujihara M, Yonehara S, Takemoto T, Tsuya T, et al. Lack of tumorigenicity of aminopyrine orally administered to B6C3F1 mice. Jpn J Cancer Res 1990;81:122-8.
- Taylor HW, Lijinsky W. Tumor induction in rats by feeding aminopyrine or oxytetracycline with nitrite. Int J Cancer 1975;16:211-5.
- El-Sheikh AH, Al-Quse RW, El-Barghouthi MI, Al-Masri FS. Derivatization of 2-chlorophenol with 4-amino-anti-pyrine: A novel method for improving the selectivity of molecularly imprinted solid phase extraction of 2-chlorophenol from water. Talanta 2010;83:667-73.
- Dou Y, Mi H, Zhao L, Ren Y. Determination of compound aminopyrine phenacetin tablets by using artificial neural networks combined with principal components analysis. Anal Biochem 2006;351:174-9.
- Dimitrova B, Doytchinova I, Zlatkova M. Determination of compound aminopyrine phenacetin tablets by using artificial neural networks combined with principal components analysis. J Pharm Biomed Anal 2000;23:955-64.
- Abd-El-Maeboud KH, El-Naggar T, El-Hawi EM, Mahmoud SA, Abd-El-Hay S. Rectal suppository: commonsense and mode of insertion. Lancet 1991;338:798-800.
- Dinc E, Baleanu D, Onur F. Spectrophotometric multicomponent analysis of a mixture of metamizol, acetaminophen and caffeine in pharmaceutical formulations by two chemometric techniques. J Pharm Biomed Anal 2001;26:949-57.
- Altun ML. HPLC Method for the analysis of paracetamol, caffeine and dipyrone. Turk J Chem 2002;26:521-8.
- Geisslinger G, Böcker R, Levy M. High -performance liquid chromatographic analysis of dipyrone metabolites to study their formation in human liver microsomes. Pharm Res 1996;13:1272-5.
- Vlahov V, Badian M, Verho M, Bacracheva N. Pharmacokinetics of metamizol metabolites in healthy subjects after a single oral dose of metamizol sodium. Eur J Clin Pharmacol 1990;38:61-5.
- Pallas Ver.: 3.6.2.1 Compudrug International Inc, Bal Harbor, FL, USA, http://www.compudrug.com.
- ICH, Q2(R1), Validation of Analytical Procedures: Text and Methodology. In: Proceedings of the International Conference on Harmonization, Geneva, June, 2005.
- LoBrutto R, Patel T. Method validation. In: Kazakevich Y, LoBrutto R, editors. HPLC for Pharmaceutical Scientists, 1st ed. Hoboken: John Wiley & Sons; 2007. p. 455-502.