- *Corresponding Author:
- C. Roy
Department of Chemistry, National Institute of Technology, Durgapur-713 209
E-mail: chinmoyanalyst@gmail.com
| Date of Submission | 29 September 2012 |
| Date of Revision | 19 February 2013 |
| Date of Acceptance | 01 March 2013 |
| Indian J Pharm Sci 2013;75(2):197-204 |
Abstract
A sensitive, fast, and stability-indicating isocratic reverse-phase ultra-performance liquid chromatography method was developed and validated for quantitative simultaneous determination of sodium methylparaben, sodium propylparaben and ketorolac tromethamine in topical dosage forms. Separation of all peaks was achieved by using acquity ethylene bridged hybrid C18 (50×2.1 mm, 1.7 μ) as stationary phase, mobile phase used was triethylamine buffer (pH 2.5):tetrahydrofuran:methanol (665:35:300, v/v/v) with isocratic mode at a flow rate of 0.40 ml/min. All component were detected at 252 nm with 10 min run time. The described method was found to be linear in the concentration range of 248-744 μg/ml for ketorolac tromethamine, 20.8-62.4 μg/ml for sodium methylparaben and 2.38-7.13 μg/ml for sodium propylparaben with correlation coefficients more than 0.999. Method was validated in terms of specificity, linearity, accuracy, precision, solution stability, filter equivalency, and robustness as per International Conference on Harmonization guideline. Formulation was exposed to the stress conditions of peroxide, acid, base, thermal, and photolytic degradation and proven all components were well separated in the presence of degradants.
Keywords
Ketorolac tromethamine, sodium methylparaben, sodium propylparaben, ultra‑performance liquid chromatography
Ketorolac tromethamine (KTR) is a nonsteroidal antiinflammatory drug in the family of heterocyclic acetic acid derivatives. Chemically it is 5‑benzoly‑2,3‑dihydro‑1H‑pyrrolizine‑1‑carboxylic acid, 2‑amino‑2‑(hydroxymethyl)‑1,3‑propanediol, often used as an analgesic, antipyretic and antiinflammatory agent. KTR acts by inhibiting the biological synthesis of prostaglandins. KTR is available in pharmaceutical dosage forms such as tablets, capsules, injections, eye‑drops, and topical gel [1‑4].
Some organic acids and their esters are commonly used as preservatives, but more often combinations of preservatives as antimicrobial agents in cosmetic, food, and pharmaceutical products are used to prevent chemical alteration and degradation of the product formulation [5].
The preservative system is an important part of semisolid formulations in preventing the deterioration of formulations from microbial contamination. Sodium methylparaben (SMP), sodium propylparaben (SPP) are the most commonly used preservatives and have been used for many years [5‑7].
A detailed literature survey revealed that a number of methods are available for determination of KTR, SMP and SPP individual in serum by high‑performance liquid chromatography (HPLC) [8‑17], gas chromatography mass spectrometry [18], spectrophotometry [19,20], liquid chromatography mass spectrometry [21], and in pharmaceutical dosage form by HPLC [22‑25].
To the best of our knowledge, there is no stability‑indicating liquid chromatography (LC) method reported for the simultaneous estimation of KTR, SMP and SPP in topical dosage forms. Therefore, attempts were made in this study to develop a fast, sensitive, selective, and stability‑indicating reversephase ultra-performance liquid chromatography (RPUPLC) method for the simultaneous determination KTR, SMP, and SPP in topical dosage forms. The proposed method was able to separate KTR, SMP, and SPP from each other and from its degradation products and placebo components. The developed LC method was validated with respect to specificity, linearity, precision, accuracy, solution stability, filter equivalency, and robustness. Force degradation studies were performed on the placebo and drug product. Developed method separated all degradation products from KTR, SMP, and SPP and exhibits stability indicating nature.
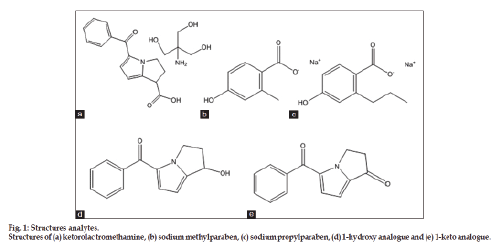
The drug product stability guideline Q1A (R2) issued by the International Conference on Harmonization (ICH) [26] suggests that stress studies should be carried out on a drug to establish its inherent stability characteristics, leading to identification of degradation products and, hence, support the suitability of the proposed analytical procedures. It also requires that analytical procedures for testing the stability of samples should be stability‑indicating and fully validated. Chemical structures of KTR, SMP, and SPP and impurities of KTR (1‑hydroxy analog and 1‑keto analogue) are presented in fig. 1.
Materials and Methods
KTR gel sample, placebo, working standards and impurities standards were provided by Dr. Reddy’s Laboratories Ltd., Hyderabad, India. HPLC grade methanol, tetrahydrofuran, triethylamine and orthophosphoric acid were used of Rankem, New Delhi, India. 0.22 μm polyvinylidene difluoride (PVDF) membrane filter, 0.2 μm PVDF syringe filter and 0.2 μm Nylon syringe filter were used of Millipore, India. Water for UPLCTM was generated using the Milli‑Q Plus water purification system (Millipore, Milford, MA, USA). UPLCTM (Acquity Waters, with auto sampler, binary solvent manager and photo‑diode array detector). Empower version 2 software (Waters) installed on a Pentium computer (Lenovo) was used for data handling. Photo‑stability chamber (Sanyo, Leicestershire, UK), dry air oven (Cintex, Mumbai, India), Cintex digital water bath were used for specificity study.
Chromatographic conditions
All chromatographic experiments were performed in the isocratic mode. Separation was achieved on ethylene bridged hybrid (BEH) C18 (50×2.1mm, 1.7 μ) column as stationary phase and using a mixture of triethylamine buffer (pH 2.5):tetrahydrofuran:methanol (665:35:300, v/v/v) as mobile phase. Other parameters such as run time (10 min), flow rate (0.4 ml/min), injection volume (2 μl), column temperature (40°) were finalized during development. KTR, SMP and SPP were detected at 252 nm. Mixture of methanol:water (45:55, % v/v) was used as diluent.
Standard solution preparation
The stock solutions of SMP (400 μg/ml) and SPP (225 μg/ml) were prepared by dissolving an appropriate amount of analyte in a mixture of methanol:water (45:55% v/v) separately. Standard solution was prepared by taking an appropriate amount of KETR and adding stock solution of SMP and SPP into it. In standard solution, concentration of KETR, SMP, and SPP were 500 μg/ml, 40 μg/ml and 4.5 μg/ml, respectively.
Sample preparation
An accurately weighed sample equivalent to 50 mg of KTR was added into a 100 ml volumetric flask. About 70 ml of diluent (mixture of methanol:water, 45:55% v/v) was added to this volumetric flask and sonicated in an ultrasonic bath for 15 min with intermittent shaking. Diluted to the volume with diluents and mixed well. Centrifuged a portion of sample at 5000 rpm for 15 min and filtered a portion of solution through 0.2 μm Nylon syringe filter.
Method development
Prime objective of this RP‑UPLC method development for determination of KTR, SMP and SPP in topical dosage form was that the developed method should be able to determine assay of drug and preservative in a single run and should be accurate, reproducible, robust, and stability indicating. All degradation products from stress conditions should be well separated from each other and method should be simple to become useful in analytical research and quality control laboratory for routine use.
KTR acid degradation sample was used for method development to optimize chromatographic conditions. All impurities (1‑hydroxy analog and 1‑keto analogue) were spiked in KTR in a way to achieve 2 μg/ml for each impurity and 500 μg/ml for KTR.
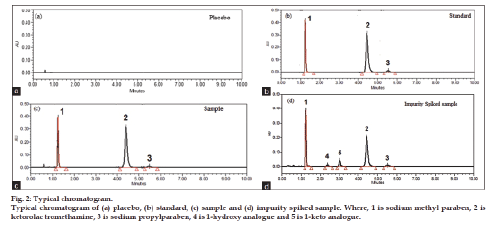
Column selection and mobile phase selection were carried out simultaneously. A method development was started with high strength silica (HSS) C18 100×2.1 mm, 1.7 μ column as stationary phase. By using mobile phase as buffer (0.1% triethyl amine, pH 2.5 adjusted by orthophosphoric acid):methanol, 50:50% v/v at a flow rate 0.4 ml/min and column temperature 40°. Peak tailing with poor resolution between KTR and SPP was observed. Different ratio of mobile phase composition, i.e. buffer (0.1% triethyl amine, pH adjusted to 2.5 by orthophosphoric acid):methanol, 70:30% v/v was tried for better separation. Good separation between KTR and SPP peaks was observed, but acid degradant peak was eluted late. Further, trials were taken with different columns such as BEH C18 (50×2.1 mm, 1.7 μ), BEH C18 (100×2.1 mm, 1.7 μ), and HSS C18 (100×2.1 mm, 1.7 μ) but with same mobile phase. Forced degradation samples were injected with triethylamine buffer (pH 2.5):tetrahydrofuran:methanol (665:35:300, v/v/v), isocratic mode, flow rate was 0.4 ml/min, column temperature 40° and run time 10 min. Furthermore in case of HSS C18 (100×2.1 mm, 1.7 μ) late elution of acid degradant was observed with bad peak shapes of all three components. In case of BEH C18 (100×2.1 mm, 1.7 μ) late elution of acid degradant was observed but peak shape was found good for all three components. Further, trial was taken to reduce run time with BEH C18 (50×2.1 mm, 1.7 μ) column. Separation of KTR, SMP, SPP and impurities peaks were found to be well separated with run time of 10 min and the representative chromatogram is shown in fig. 2.
KTR, SMP, and SPP are soluble in methanol and in water. Based on solubility different ratio of methanol and water were tried as diluent for sample dispersion and extraction of all three components. Good recovery of all components was found with methanol:water (45:55, v/v) as diluent.
Analytical method validation
After satisfactory development of the method, it was subjected to method validation as per ICH guideline [27]. The method was validated to demonstrate its suitability for intended purpose by the standard procedure. Analytical method validation was carried out by means of system suitability, accuracy, precision, linearity, robustness, solution stability, forced degradation study, and filter compatibility.
System suitability parameters were measured so as to verify the system, method and column performance. System suitability test parameters were checked by repetitively injecting the drug solution specific concentration levels of KTR (500 μg/ml), SMP (40 μg/ml) and SPP (4.5 μg/ml) to check the reproducibility of the system.
Specificity/forced degradation study
Specificity is the ability of the method to measure the analyte response in the presence of its potential impurities [27]. Forced degradation studies were performed to demonstrate selectivity and stabilityindicating capability of the proposed RP‑UPLC method. Placebo, standard, and sample preparation were injected to check the interference study.
Forced degradation by peroxide oxidation was carried out by weighing sample equivalent to 50 mg of KTR into 100 ml volumetric flask. About 30 ml of diluent was added to this volumetric flask and sonicated in an ultrasonic bath for 15 min with intermittent shaking. Then 5 ml of 30% v/v H2O2 was added and heated on water bath at 70° for 2 h. Cooled to room temperature, diluted to the volume with diluent and mixed well. A portion of sample was centrifuged at 5000 rpm for 15 min and filtered through 0.2 μm Nylon syringe filter.
Forced degradation through acid hydrolysis was carried out by weighing sample equivalent to 50 mg of KTR into 100 ml volumetric flask. About 30 ml of diluent was added to this volumetric flask and sonicated in an ultrasonic bath for 15 min with intermittent shaking. Then 5 ml of 1 N HCl was added and heated on water bath at 70° for 1 h. cooled to room temperature and neutralized with 1 N NaOH. It was diluted to the volume with diluent and mixed well. Centrifuged a portion of sample at 5000 rpm for 15 min and filtered through 0.2 μm Nylon syringe filter.
Forced degradation through base hydrolysis was carried out by weighing sample equivalent to 50 mg of KTR into 100 ml volumetric flask. About 30 ml of diluent was added to this volumetric flask and sonicated in an ultrasonic bath for 15 min with intermittent shaking. Then 5 ml of 4 N NaOH was added and kept at room temperature (RT) for 15 min, followed by cooling to room temperature and neutralizing with 4 N HCl. Diluted to the volume with diluent, mixed well. A portion of sample was centrifuged at 5000 rpm for 15 min and filtered through 0.2 μm Nylon syringe filter.
Photolytic degradation was studied by exposing sample to 1.2 million lux h and further followed by sample preparation procedure as mentioned above. Thermal exposed sample was studied by exposing the sample at 105° for 6 h followed by sample preparation procedure as mentioned above.
Precision
The precision of the assay method was verified by repeatability and by intermediate precision. Precision was investigated using the sample preparation procedure for six real gel samples and analyzing by the proposed method. Intermediate precision was studied using different column and performing the analysis on different day.
Accuracy
To confirm the accuracy of the proposed method, recovery experiments were carried out by standard addition technique. Three different levels (50, 100 and 150%) of standards were added to preanalyzed placebo samples in triplicates. The percentage recoveries of KTR, SMP and SPP at each level and each replicate were determined. The mean of percentage recoveries (n=3) and the % relative standard deviation (RSD) were calculated.
Linearity
Linearity was demonstrated from 50 to 150% of standard concentration KTR (500 μg/ml), SMP (40 μg/ml) and SPP (4.5 μg/ml) using minimum five calibration levels (50, 75, 100, 125 and 150%) for the KTR, SMP and SPP compounds, which gave a good confidence on analytical method with respect to linear range.
Robustness
The robustness as a measure of method capacity to remain unaffected by small, but deliberate changes in chromatographic conditions was studied by testing the influence of small changes in flow rate (±0.04 ml/ min), change in column oven temperature (±2°), change in mobile phase buffer pH (±0.2), change in mobile phase organic composition (±2%) of tetrahydrofuran and methanol.
Stability
Stability of sample solution was established by storage of sample solution at ambient temperature for 24 h. Sample solution was re‑analyzed after 12 and 24 h time intervals and the assay was determined and compared against fresh sample.
Filter compatibility
Filter compatibility was performed for nylon 0.2 μm syringe filter (Millipore) and PVDF 0.2 μm syringe filter (Millipore). To confirm the filter compatibility in the proposed method, filtration recovery experiment was carried out by sample filtration technique. Sample was filtered through both syringe filters and % assay for both syringe filters were determined and compared against centrifuged sample.
Results and Discussion
The % RSD of the area of KTR, SMP, and SPP from five replicate injections was below 0.20%. Low values of % RSD of replicate injections indicate that the system is precise. Results of other system suitability parameters such as theoretical plates and tailing factor are presented in Table 1.
| Parameters | KTR | SMP | SPP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | T | % RSD* | N | T | % RSD* | N | T | % RSD* | |||
| Precision | 5705 | 1.2 | 0.17 | 4424 | 1.3 | 0.17 | 5882 | 1.2 | 0.19 | ||
| Intermediate precision | 6243 | 1.2 | 0.34 | 4945 | 1.3 | 0.32 | 6481 | 1.2 | 0.40 | ||
| At 0.44 ml/min flow rate | 5925 | 1.2 | 0.39 | 4522 | 1.3 | 0.41 | 5934 | 1.3 | 0.48 | ||
| At 0.36 ml/min flow rate | 6453 | 1.2 | 0.33 | 4865 | 1.3 | 0.37 | 6458 | 1.3 | 0.39 | ||
| At 42° column temperature | 6529 | 1.3 | 0.41 | 4438 | 1.3 | 0.23 | 6501 | 1.3 | 0.23 | ||
| At 38° column temperature | 5790 | 1.3 | 0.23 | 4598 | 1.3 | 0.23 | 5934 | 1.3 | 0.26 | ||
| Mobile phase buffer pH 2.7 | 5847 | 1.3 | 0.38 | 4906 | 1.3 | 0.34 | 6040 | 1.3 | 0.35 | ||
| Mobile phase buffer pH 2.3 | 5635 | 1.2 | 0.36 | 4584 | 1.3 | 0.29 | 6789 | 1.3 | 0.43 | ||
| MP (buffer: THF: Methanol; | 4255 | 1.3 | 0.38 | 4502 | 1.3 | 0.12 | 5476 | 1.3 | 0.27 | ||
| 665:55:300) | |||||||||||
| MP (buffer: THF: Methanol; | 4511 | 1.3 | 0.48 | 4906 | 1.3 | 0.21 | 5509 | 1.3 | 0.26 | ||
| 665:15:300) | |||||||||||
| MP (buffer: THF: Methanol; | 5652 | 1.3 | 0.26 | 4854 | 1.3 | 0.25 | 5855 | 1.3 | 0.22 | ||
| 665:35:280) | |||||||||||
| MP (buffer: THF: Methanol; | 6060 | 1.3 | 0.19 | 4673 | 1.3 | 0.21 | 6643 | 1.3 | 0.20 | ||
| 665:35:320) | |||||||||||
Table 1: System Suitability Results (Precision, Intermediate Precision And Robustness)
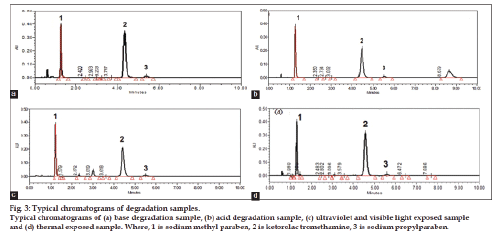
Fig. 2 shows that there was no interference at the retention time of KTR, SMP and SPP due to blank, placebo, impurities or degradation products. Placebo, standard, and sample chromatograms are presented in figs. 2a‑c, respectively. Significant degradation was not observed when KTR was subjected to hydrogen peroxide oxidation (30% H2O2, 70°, 2 h). Whereas, significant degradation was observed when the drug product was subjected to acid hydrolysis (1 N HCl, 70°, 1 h) and base hydrolysis (4 N NaOH, RT 15 min). Sample chromatogram for base degradation and acid degradation study are presented in figs. 3a and b, respectively. Furthermore, significant degradation was observed when the drug product was subjected to photolytic (1.2 million lux h) and thermal (105º, 6 h) degradation. Chromatograms of photolytic degradation and thermal degradation study are presented in figs. 3c and d, respectively. Peak due to KTR, SMP, and SPP were investigated for spectral purity in the chromatogram of all exposed samples and found to be spectrally pure. Peak purity angle were less than peak purity threshold for KTR, SMP, and SPP. The purity and assay of KTR was unaffected by the presence of its impurities and degradation products and thus, confirmed the stability‑indicating nature of the developed method. Results from forced degradation study are given in Table 2.
Figure 3: Typical chromatograms of degradation samples.
Typical chromatograms of (a) base degradation sample, (b) acid degradation sample, (c) ultraviolet and visible light exposed sample
and (d) thermal exposed sample. Where, 1 is sodium methyl paraben, 2 is ketorolac tromethamine, 3 is sodium propylparaben.
| Stress conditions | Ketorolac tromethamine | Sodium Methylparaben | Sodium Propylparaben | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PA | PTH | % Deg. | PA | PTH | % Deg. | PA | PTH | % Deg. | |||
| Acidic hydrolysis (1 N HCl, 70°, 60 min) | 0.634 | 1.034 | 32.5 | 0.217 | 1.053 | 5.9 | 0.653 | 2.063 | 5.0 | ||
| Alkaline hydrolysis (4 N NaOH, RT, 15 min) | 0.441 | 1.006 | 6.8 | 0.577 | 1.020 | 13.1 | 0.857 | 2.392 | 6.2 | ||
| Oxidation (30% H2O2, 70°, 2 h) | 0.150 | 1.011 | ND | 0.502 | 1.073 | ND | 0.548 | 2.508 | ND | ||
| Thermal (At 105°, 6 h) | 0.237 | 1.017 | 8.8 | 0.250 | 1.054 | 9.2 | 0.665 | 2.574 | 6.7 | ||
| UV and visible light exposed | 0.625 | 1.037 | 36.3 | 0.434 | 1.049 | 6.0 | 0.728 | 2.016 | 4.1 | ||
Table 2: Results Of Forced Degradation Study
The precision of the assay method was verified by repeatability and by intermediate precision. The samples in gel preparation were analysed with six replication and % assay of SMP, SPP and KTR were 102.4, 99.2 and 98.8, respectively with RSD below 1.5%. Intermediate precision was studied using different column, and performing the analysis on different day. Results are presented in Table 3 along with precision data. Low values of % RSD, indicates that the method is precise.
| Components | Precision* (day-1) | Intermediate* precision (day-2) | ||
|---|---|---|---|---|
| % Assay | % RSD | % Assay | % RSD | |
| Ketorolac tromethamine | 102.4 | 1.20 | 100.0 | 1.00 |
| Sodium methylparben | 99.2 | 0.91 | 99.1 | 0.91 |
| Sodium propylparben | 98.8 | 0.90 | 98.9 | 0.97 |
Table 3: Method Precision
The amount recovered was within ±1% of the amount added, which indicates that the method is accurate and also there is no interference due to excipients present in topical formulation. The results of recoveries for assay are shown in Table 4. The response was found to be linear for all KTR, SMP and SPP from 50 to 150% of standard concentration and correlation coefficient was also found greater than 0.999. Bias was also found within ±0.5. The result of correlation coefficients, Y‑intercept, bias and linearity equations for KTR, SMP and SPP are presented in Table 5.
| Components | At 50% | At 100% | At 150% |
|---|---|---|---|
| Ketorolac tromethamine | |||
| % Recovery | 99.1 | 99.1 | 100.2 |
| % RSD | 0.62 | 0.48 | 0.54 |
| Sodium methylparben | |||
| % Recovery | 98.9 | 99.1 | 100.4 |
| % RSD | 0.20 | 0.20 | 0.43 |
| Sodium propylparben | |||
| % Recovery | 98.9 | 98.6 | 100.0 |
| % RSD | 0.43 | 0.10 | 0.51 |
Table 4: Accuracy Results
| Components | Linearity range (µg/ml) | Correlation coefficient (r) | Linearity (equation) | Y-intercept bias (%) |
|---|---|---|---|---|
| Ketorolac tromethamine | 248-744 | 0.99991 | y=5417.16x+7292.20 | 0.269 |
| Sodium methylparben | 20.8-62.4 | 0.99993 | y=28889.33x−5653.40 | −0.470 |
| Sodium propylparben | 2.38-7.13 | 0.99986 | y=23684.55x−359.20 | −0.318 |
Table 5: Linearity Results
In case of robustness study, no significant effect was observed on system suitability parameters such as theoretical plates, tailing factor and % RSD of all three components, when small, but deliberate changes were made to chromatographic conditions. The results are presented in Table 1 along with system suitability parameters of precision and intermediate precision study. Thus, the method was found to be robust with respect to flow rate (±0.04 ml/min), change in column oven temperature (±2°), change in mobile phase buffer pH (±0.2) and change in mobile phase composition (±2%) of tetrahydrofuran and methanol.
Sample solution did not show any appreciable change in assay value when stored at ambient temperature up to 24 h, which are presented in Table 6. The results from solution stability experiments confirmed that sample solution was stable for 24 h during assay determination.
| % Assay | Initial | After 12 h | After 24 h |
|---|---|---|---|
| Ketorolac tromethamine | 102.7 | 101.5 | 101.1 |
| Sodium methylparben | 99.8 | 98.6 | 98.1 |
| Sodium propylparben | 99.2 | 98.7 | 98.1 |
Table 6: Solution Stability
There was no significant change in assay of nylon, PVDF and centrifuged samples. Assay results as a percentage are presented in Table 7. In displayed result, difference in % assay was not observed more than ±0.2, which indicates that both syringe filters have a good compatibility with sample solution.
| % Assay | Centrifuged sample | PVDF syringe filter | Nylon syringe filter |
|---|---|---|---|
| Ketorolac tromethamine | 102.4 | 101.2 | 100.9 |
| Sodium methylparben | 99.2 | 99.5 | 99.0 |
| Sodium propylparben | 98.8 | 99.2 | 98.5 |
Table 7: Filter Compatibility
A fast, sensitive, and stability indicating RP‑UPLC method was successfully developed for the determination of KTR, SMP and SPP in topical dosage form. The method validation results have established that the method is selective, precise, accurate, linear, robust, filter compatible, and stability indicating. The run time (10.0 min) enables rapid determination of all three components. Moreover, it may be applied for determination of KTR, SMP and SPP in the study of content uniformity, tube homogenity and in vitro release test profiling of KTR topical dosage forms, where sample load is higher and high throughput is essential for faster delivery of results.
Acknowledgments
The authors would like to thank M/s Dr. Reddy’s Laboratories Ltd. for supporting this work. The authors also would like acknowledge Mr. Ajay Vairale for his immense support. The authors’ Intellectual Property Management department (IPM) has given this manuscript internal publication number PUB 00185‑12.
References
- Available from: http://www.drugsupdate.com/brand/generic/ Ketorolac/10464. [Accessed on 2012 Sep 29].
- Dubey R, Bommagani M, Venkateswarlu V, Mullangi R, Karnati HR, Thammera RK, et al. Ketorolac tromethamine transdermal gel: Development, in vitro and in vivo evaluation. J Pain Palliat Care Pharmacother 2009;23:26-34.
- Vodithala S, Khatry S, Shastri N, Sadanandam M. Formulation and evaluation of ion activated ocular gels of ketorolac tromethamine. Int J Chromatogr Pharm Res 2010;2:33-8.
- Rajveer CH, Rathinaraj BS, Fareedullah M, Bangale GS, Shinde G. Design and evaluation of ketorolac tromethamine sustained release matrix tablets. Int J Curr Res Rev 2010;2:39-55.
- Shabir GA. Development and validation of a stability-indicating LC method for the determination of domperidone, sorbic acid and propylparaben in pharmaceutical formulations. J LiqChromatogrRelatTechnol 2010;33:1802-13.
- Kuang LK, You-Zung H. Determination of preservatives in food products by cyclodextrin-modified capillary electrophoresis with multiwavelength detection. J Chromatogr A 1997;768:334-41.
- Boonleang J, Tanthana C. Simultaneous stability-indicating HPLC method for the determination of cisapride, methylparaben and propylparaben in oral suspension. Songklanakarin J SciTechnol 2010;32:379-85.
- Patri S, Patni AK, Iyer SS, Khuroo AH, Monif T, Rana S, et al. Validated HPLC-tandem MS (LC-MS/MS) method for simultaneous determination of R(+)-ketorolac and S(−)-ketorolac in human plasma and its application to a bioequivalence study. Chromatogr Res Int 2011;Article ID 214793:1-11.
- Wu AT, Massey IJ. Simultaneous determination of ketorolac and its hydroxylated metabolite in plasma by high-performance liquid chromatography. J Chromatogr 1990;534:241-6.
- Chaudhary RS, Gangwal SS, Jindal KC, Khanna S. Reversed-phase high-performance liquid chromatography of ketorolac and its application to bioequivalence studies in human serum. J Chromatogr 1993;614:180-4.
- Jung D, Mroszczak EJ, Wu A, Ling TL, Sevelius H, Bynum L. Pharmacokinetics of ketorolac and p-hydroxyketorolac following oral and intramuscular administration of ketorolac tromethamine. Pharm Res 1989;6:62-5.
- Vakily M, Corrigan B, Jamali F. The problem of racemization in the stereospecific assay and pharmacokinetic evaluation of ketorolac in human and rats. Pharm Res 1995;12:1652-7.
- Jamali F, Pasutto FM, Lemko C. HPLC of ketorolac enantiomers and application to pharmacokinetics in the rat. J LiqChromtogr 1989;12:1835-50.
- Hayball PJ, Tamblyn JG, Holden Y, Wrobel J. Stereoselective analysis of ketorolac in human plasma by high-performance liquid chromatography. Chirality 1993;5:31-5.
- Mills MH, Mather LE, Gu XS, Huang JL. Determination of ketorolac enantiomers in plasma using enantioselective liquid chromatography on an alpha 1-acid glycoprotein chiral stationary phase and ultraviolet detection. J Chromatogr B Biomed Appl 1994;658:177-82.
- Campanero MA, Ocariz AL, Quetglas EG, Sadaba B, Azanza JR. Determination of ketorolac enantiomers in plasma using enantioselective LC. Chromatogr 1998;48:203-8.
- Franceschi L, Furlanut M. A simple and sensitive HPLC method to monitor serum and synovial fluid concentrations of ketorolac in reumathologic patients. J Bioanal Biomed Anal 2010;2:121-4.
- Logan BK, Friel PN, Peterson KL, Predmore DB. Analysis of ketorolac in postmortem blood. J Anal Toxicol 1995;19:61-4.
- Kamath BV, Shivram K, Shah AC. Determination of diclofenac sodium, famotidine and ketorolac tromethamine by flow injection analysis using dichloronitrophenol. J Pharm Biomed Anal 1994;12:343-6.
- Fegade JD, Mehta HP, Chaudhari RY, Patil VR. Simultaniousspectrophotometric estimation of ofloxacin and ketorolac tromethaminein ophthalmic dosage form. Int J Chem Tech Res 2009;1:189-94.
- Ing-Lorenzini KR, Desmeules JA, Besson M, Veuthey JL, Dayer P, Daali Y. Two-dimensional liquid chromatography-ion trap mass spectrometry for the simultaneous determination of ketorolac enantiomers and paracetamol in human plasma: Application to a pharmacokinetic study. J Chromatogr A 2009;1216:3851-6.
- Razzaq SM, Ashfaq M, Khan IU, Mariam I. Stability indicating HPLC method for the simultaneous determination of ofloxacin and ketorolac tromethamine in pharmaceutical formulations. Anal Methods 2012;4:2121-6.
- Razzaq SM, Mariam I, Khan IU, Ashfaq M. Development and validation of LC method for gatifloxacin and ketorolac tromethamine in combined dosage form. J Liq Chromatogr Relat Technol 2012;35:651-61.
- Demircan S, Sayın F, Basc NE, Unlu N, Kır S. Determination of ketorolac tromethamine in human eye samples by HPLC with PDA detection. Chromatogr 2007;66:135-9.
- Gandla K, Kumar JM, Bikshapathi DB, Spandana R. A validated RP-HPLC method for simultaneous estimation of febuxostat and ketorolac tromethamine in pharmaceutical formulations. J Drug DelivTher 2012;2:173-6.
- ICH Q1A (R2). Stability Testing of New Drug Substances and Products. Geneva: International Conference on Harmonization; 2003.
- ICH Q2 (R1). Validation of Analytical Procedures: Text and Methodology. Geneva: International Conference on Harmonization; 2005.