- *Corresponding Author:
- Brinda Durai Raj
Department of Biochemistry, PSG College of Arts and Science, Coimbatore, Tamil Nadu 641014, India
E-mail: publicationbiochemistry@gmail.com
| Date of Received | 04 August 2022 |
| Date of Revision | 02 May 2023 |
| Date of Acceptance | 15 February 2024 |
| Indian J Pharm Sci 2024;86(1):361-368 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Autism spectrum disorder belongs to a class of neurodevelopmental abnormality associated with complex behavioural traits and complications in social and cognitive performance. One in every hundred children has estimated to have autism according to the World Health Organisation. Therefore, research on the prognosis, diagnosis and therapy of autism spectrum disorder is the current need of the hour. Zebrafish as a model organism has enabled rapid advancements in neuroscience and biological psychiatry. Zebrafish behavioural characteristics can be used to understand the neuronal networks, physiological indicators and genetic basis of brain function. The search for neuroprotective medicines with fewer side effects has risen in popularity. Based on previous evidence, chrysin exhibited neuroprotective properties, inhibits neuro-inflammation and aids in the prevention of cognitive deterioration. In the present study, the neuroprotective role of chrysin was explored against valproic acid-induced autism spectrum disorder-like behaviour. The most efficient route of valproic acid administration was determined by testing two alternative techniques were immersion and intraperitoneal injection. The primary behavioural endpoints in autism spectrum disorder are learning, memory and social behaviour were studied via behavioural analysis. The findings imply that intraperitoneal injection is a more reliable method of induction when compared to immersion since the drug's uptake is reported to be uniform in all fish. According to the experimental data, chrysin positively stimulates on zebrafish social behaviour. More research is needed to fully comprehend the neurochemical pathways behind chrysin's effects on valproic acid-induced autism-like behaviour. Finally, our research supports the use of zebrafish models to investigate the neuroprotective benefits of drugs. From our findings, it is proven that chrysin could be used to alleviate valproic acid-induced autism-like behaviour.
Keywords
Autism, chrysin, zebrafish, valproic acid, memory, social behaviour, intraperitoneal injection
Autism Spectrum Disorder (ASD) is a heterogeneous neuro-developmental disorder that appears before the age of 3 y and affects social communication, atypical and repetitive motor behaviours[1]. ASD is triggered by a combination of hereditary and environmental factors impacting the developing brain, but no specific underlying mechanism has been identified[2]. Therefore the therapy and diagnosis of ASD is challenging. Currently no pharmacological treatment for ASD is available but, behavioural therapies and the use of highly controlled learning environments are used to treat ASD[3].
The need for neuroprotective medicines to treat ASD associated symptoms is remarkably high. Many active pharmaceutical ingredients are said to be present in plants, making them natural remedies[4]. The usage of bioactive components found in plants is growing in popularity. Flavonoids are the most common type of plant secondary metabolite with a broad range of biological properties important for human health. Chrysin is a hydroxylated flavone derivative that has a variety of therapeutic properties like anti-infllamatory, anti-asthmatic activity, anti-cancer activity, anti-hypercholesteraemic, and cardio-protective properties including neuroprotective properties[5]. The B and C rings in the structure of chrysin are devoid of oxygen, which is associated with its biological effects[6]. Chrysin can be found in honey, propolis, and a variety of plants[5].
The multifaceted nature of ASD makes it difficult to establish a relevant animal model to study the neurobiology of ASD[7]. Zebrafish (Danio rerio) are increasingly being used as neurodevelopmental model[8] which has genetic resemblance to humans[9]. Zebrafish is a schooling cyprinid that belongs to the carp family[10]. They are excellent species to explore experimental, genetic and pharmacological studies primarily because of robustness in its behaviours[11]. Zebrafish are also inexpensive, easy to reproduce, and a large number of fishes can be maintained in confined spaces. Adult zebrafish demonstrate a full range of mature behaviours compared to larvae[12]. Unlike other laboratory behavioural models, zebrafish are inherently sociable animals which prefer to be in the company of their peers. Therefore zebrafish is an ideal model for studying the genetics of social behaviour[13].
With this background, we aimed to investigate the therapeutic potential of chrysin against valproic acid induced autism-like behaviour in zebrafish in the experimental paradigm of ASD. This also includes establishing a reliable approach of induction method by comparing two different modes of administration of valproic acid-immersion and intraperitoneal injection.
Materials and Methods
Zebrafish maintanence:
At the aquarium shop in Coimbatore, 3-4 mo old adult zebrafish (Danio rerio) of the heterogeneous wild-type strain were purchased. All zebrafish were kept in a standardised husbandry environment at the animal facility. The zebrafish tanks were kept at a temperature of 26°-30°, a pH of 6.8 to 7.1, and a light intensity of 250 lx with a cycle of 14 h of light and 10 h of darkness. A timer was used to turn on the lights at 8 AM and turn them off at 10 PM. 3 times a d, the zebrafish were fed quality food. The zebrafish were housed in standard zebrafish tanks with a length of 36 cm, a width of 26 cm, and a height of 22 cm. A water circulation system was installed in the tanks to ensure regular aeration. Females and males were housed separately in group housing, which consisted of ten to twelve fish per tank. All animal experiments were carried out in conformity with Organization for Economic Cooperation and Development guidelines. All the experimentation on fish were carried out according to the Guidelines of Control and Supervision of Experimentation on Animals (CPCSEA) for experimentation on fishes, Government of India, Ministry of Fisheries, Animal Husbandry and Dairying, Department of Animal Husbandry and Dairying, Committee for the Purpose of CPCSEA, 2021.
Chemicals:
Valproic acid and chrysin used in the experiments were purchased from Sigma Aldrich.
Drug treatment and grouping
The effects of two administration routes of valproic acid, immersion and intraperitoneal injection, in inducing autism-like behaviour in adult zebrafish were compared. We investigated its effects by observing and grading zebrafish behaviour. The neuroprotective properties of chrysin against valproic acid-induced changes were also investigated.
There were five experimental groups. Group I indicates normal/control; group II indicates valproic acid (Immersion technique), group III indicates valproic acid (Intraperitoneal injection-100 mg/kg), group IV indicates valproic acid (immersion) and chrysin (the injection volume was 1 µl/0.1 g of body weight) and group V indicates valproic acid (Intraperitoneal injection) and chrysin (the injection volume was 1 µl/0.1 g of body weight). The chemicals were prepared using dimethyl sulfoxide and dosing was followed according to Choo et al.[14] and German-Ponciano et al.[15].
Each group consists of 20 fish and all tests were performed in triplicates. The zebrafish were individually submerged in an anaesthetic solution (30 mg/l Benzocaine) until they stopped moving before each intraperitoneal injection. When numerous intraperitoneal injections were needed on the same zebrafish at the same time, the injections were delivered at alternating lateral ends rather than in the midline between the pelvic fins.
Behavioural analysis:
Novel tank diving test: The locomotor and exploratory activities of fish were monitored using a novel tank diving test to determine if chrysin inhibits valproic acid-induced hyperlocomotion. Individual zebrafish were subjected to non-chlorinated water or chrysin for 60 min before being injected with valproic acid intraperitoneally/immersion. 15 min after valproic acid injection/immersion, behaviour studies were conducted. This test protocol was performed following Franscescon et al.[16]. The distance travelled immobile episodes and time spent in the top and bottom areas were assayed.
In pharmaceutical research, the open field activity test in zebrafish is widely employed to assess anxiolytic behaviour which is suggested by swimming in the centre of a circular open field. This test methodology was carried out according to Sison et al.[17]. The time spent in the periphery and centres is taken into account in the open tank test.
Light vs. dark preference test: The protocol of this test has been performed following Magno et al.[18]. Scototaxis is a viable option to open field and unique tank tests. It's similar to the murine light/dark box, which makes use of zebrafish's natural desire to explore new environments when they're confronted with the unpleasant features of a brightly lighted environment[19]. The percentage of time present in the light region and the number of times the fish crossed the light and dark chambers were considered for analysis.
Mirror attack biting test: The mirror biting test has been modified which involves placing a mirror in the tank and introducing fish to the tank, a situation in which the mirror and tank surroundings are both novel to the fish. While this change may be less ideal for anxious fish with high baseline freezing, it may be appropriate for more active and less anxious strains (i.e., those whose behaviour is less affected by the initial novelty stimulus)[20]. The number of attacks on the mirror and the time spent in the mirror arena was recorded and decoded.
Social behaviour:
Shoaling behaviour: Between 10 AM and 12:30 PM, a shoaling test was performed using a glass dish (9 cm diameter) with 250 ml fish water and 10 fishes per dish. The Inter-Individual Distance (IID) and the Nearest Neighbour Distance (NND) among a group of zebrafish were used as shoaling behaviour assay parameters. For each group, we tracked the movement of 10 fishes for 8 min, collecting data every 15 s. The first two min were used for acclimatisation and the remaining six min were used for analysis. Each group had two trials, which were then merged (20 fishes per group) and examined and the assay was performed three times[21].
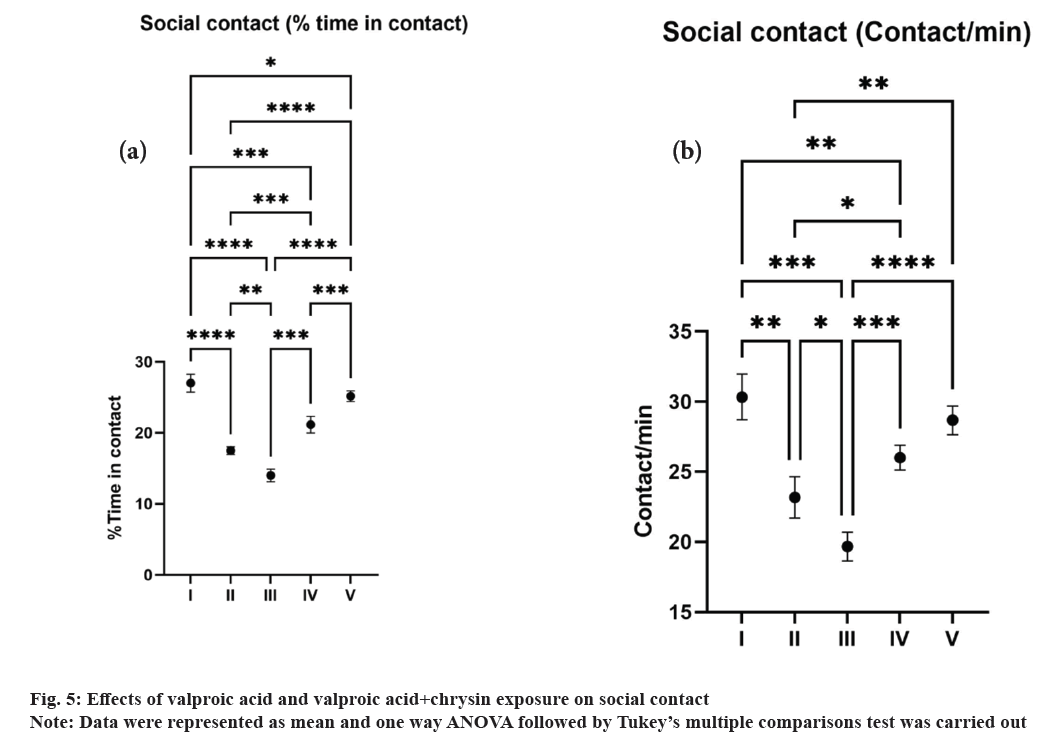
Social contact: We tracked movement for 6 min and assessed social contact behaviour using data acquired every 6 min. The average contact time and the contact duration time were noted[21].
Software: The software used for the behavioural analysis is Toxtrac[22].
Statistics:
All the experiments were statistically analysed using one way ANOVA test followed by Tukey’s multiple comparisons test. The test was used to compare differences between the treatment and control groups, all the data are reported as mean±standard error unless otherwise stated p<0.05 was set as the significant level. Graph Pad Prism 9.3.0 was used to analyse the data.
Results and Discussion
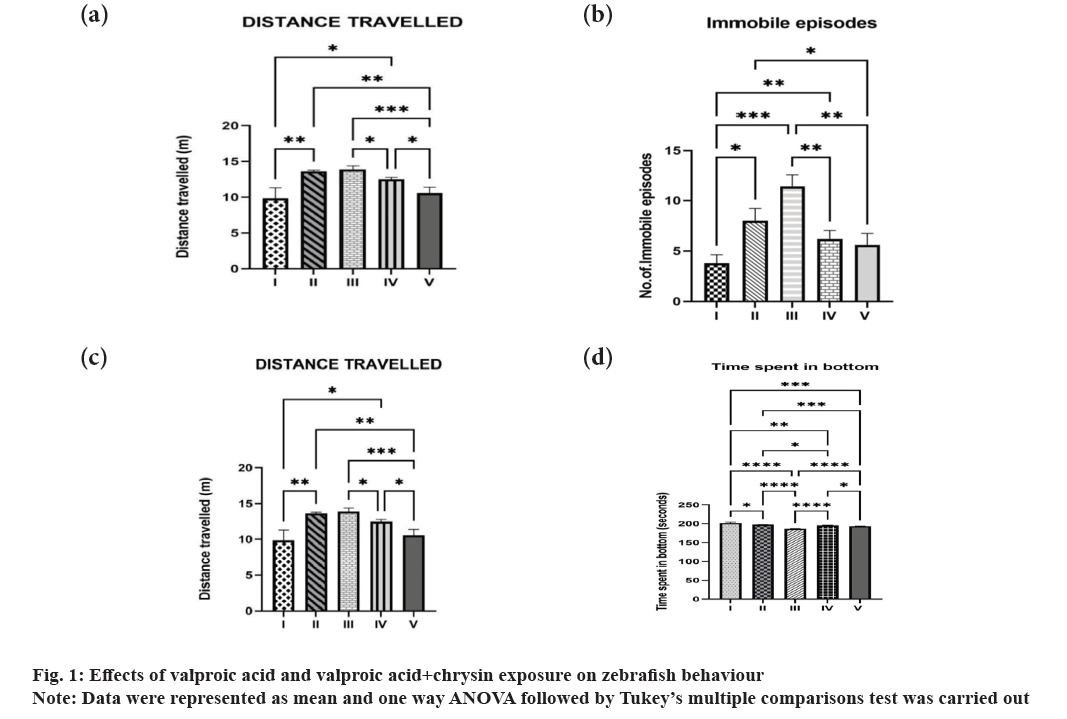
The novel tank investigation utilises the zebrafish's natural urge to find a safe place to stay when placed in unfamiliar environment. In this experiment, the fish gradually explores the upper area of the aquarium by diving to the bottom of the tank and staying there until it feels safe. The distance covered by the valproic acid exposed groups varied from 9.6-13.86 cm, with Group III travelling the longest distance of 13.86 cm. After pre-treating with chrysin, the valproic acid-treated treated group demonstrated a decline in the distance travelled, from 13.86-10.55 cm (fig. 1a).
Locomotor activity in zebrafish has been shown to be an accurate tool for determining the impact of different stimuli[23]. Zebrafish may display novelty-induced immobility, erratic movement, or increased activity due to an increased desire for exploration, similar to that observed in rodents. The distance travelled has increased, which is a sign of panic or hyperactivity and increased mobility[24].
The groups that received valproic acid intraperitoneally represented an average of 11 immobility episodes. After exposure to chrysin via immersion and intraperitoneal injection, there was a decrease in the number of immobility episodes to 6 and 5, respectively (fig. 1b). Fish at the bottom of the tank that is completely immobile are said to be "freezing." Considering the latency, frequency, length, and site of freezing, it may be the result of severe stress or anxiety[25].
In contrast to the control and chrysin pre-treated groups, zebrafish exposed to valproic acid were more inclined towards staying at the top of the tank rather than exploring other areas of the tank (fig. 1c). The period of time spent at the tank's top area suggests that zebrafish display anxiolytic behaviour[26]. The tendency to cross the centre of the tank increases as a result of escape behaviour along the side walls and corner[27].There is a decline in the time spent in the periphery in all groups when compared to group I (fig. 1d). This may be due to the anxiolytic behaviour of zebrafish suggested by swimming in the middle of a circular-shaped open field[28].
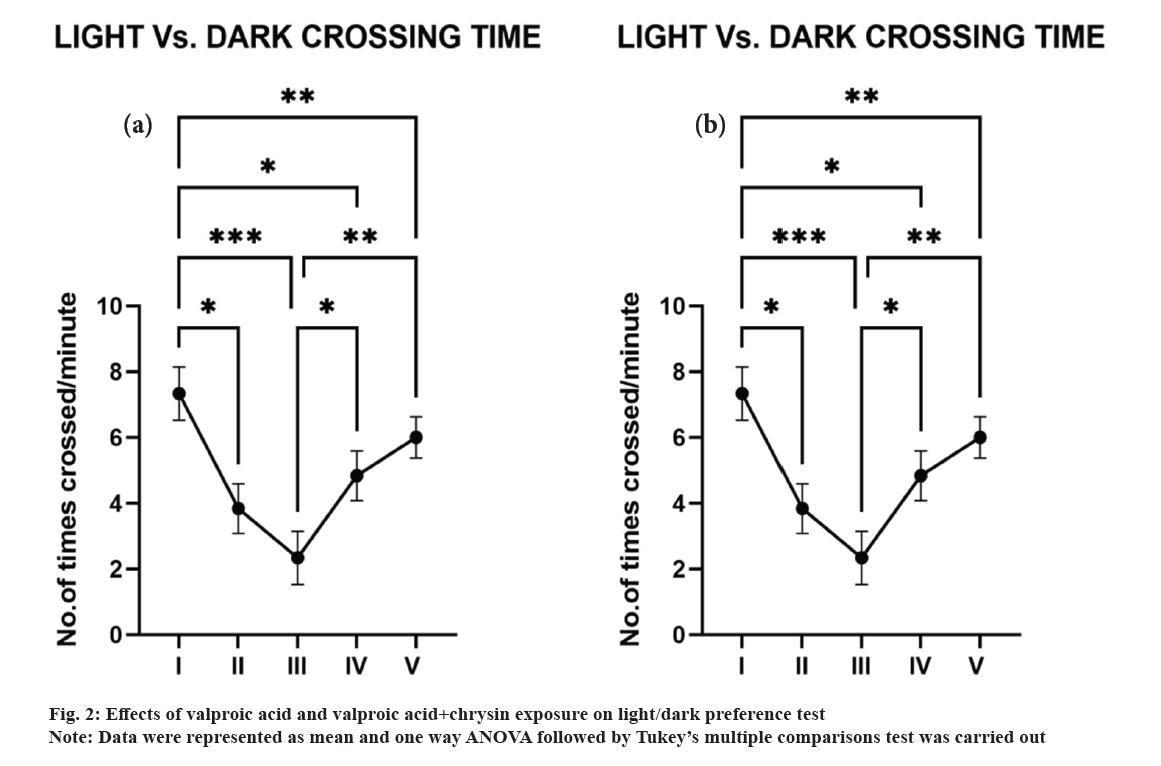
In light vs. dark experiment, the reduced transition between light stages was observed in zebrafish exposed to valproic acid and the percentage of time spent in the light area was found to be increased (fig. 2a). Animals prefer dark region when compared to light region. There was a marked decrease in the crossing time between control and other groups (fig. 2b). Fish may have chosen the dark part when the light intensity increased to reduce their visibility against the background. Zebrafish larvae can adjust the distribution of melanin pigment in their skin to suit environmental light levels and backgrounds, and this capacity lasts until they reach adulthood. At greater light levels, preferring the black half of the light vs dark chamber could enhance their visual sensitivity[29].
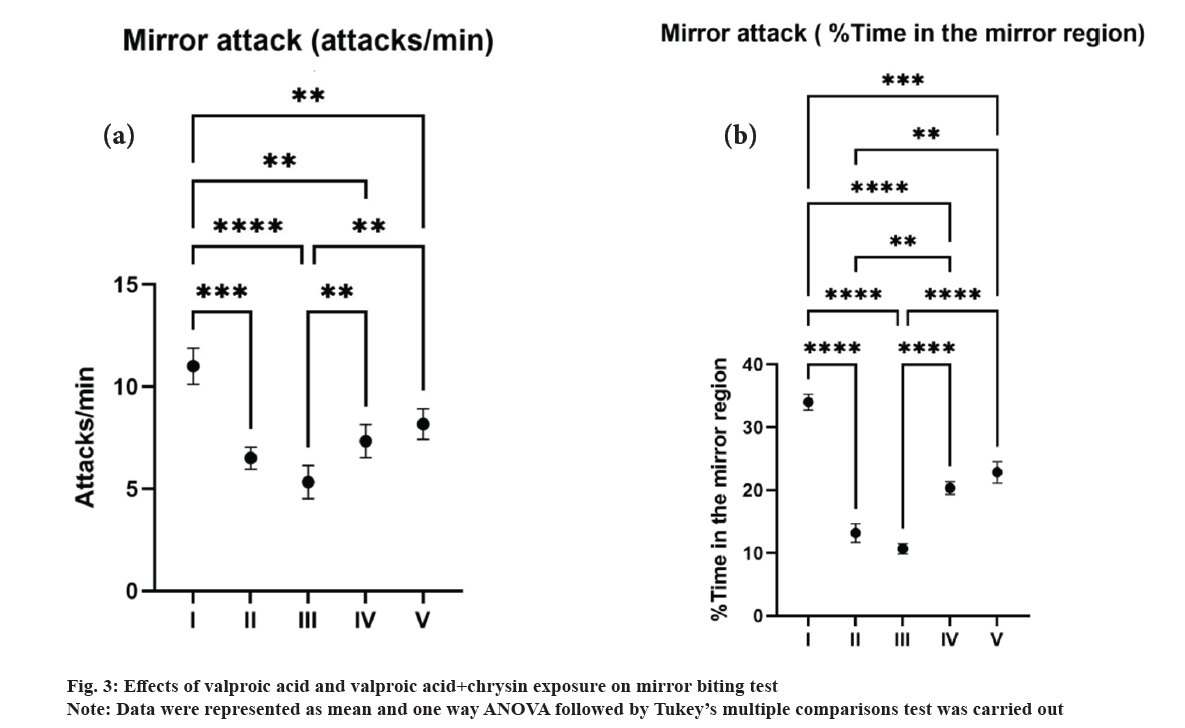
Valproic acid-exposed fish had significantly fewer attacks on the mirror (fig. 3a) and spent less time in the mirror area (fig. 3b) (mirror attack assay). The highest number of mirror attacks was observed in group I followed by a decrease in all other groups. A minimum of 5 and a maximum of 13 attacks/min were observed in group III and I respectively. Chrysin has an effect on the fish's mirror attack behaviour which is observed by an increase in mirror region. The mirror biting test can elicit both social and aggressive behaviour, which will be severely impacted if the fish have social deficits. Greater head-butting or mirror biting in this test might indicate increased zebrafish aggression and/or sociability, whereas excessively low reflexes could indicate ASD-like characteristics[30].
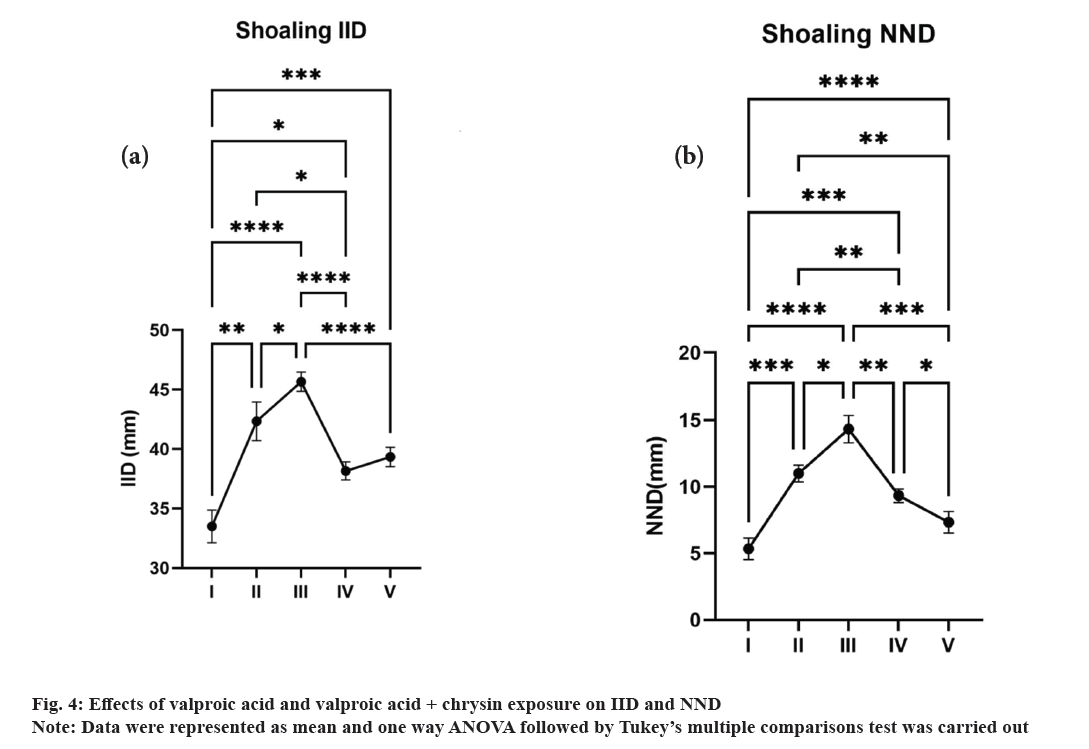
Shoaling behaviour measures Inter-Individual Distance (IID) and the Nearest Neighbour Distance (NND). Fig. 4a and fig. 4b depicts a significant increase in valproic acid exposed groups with a striking difference in chrysin treated groups. The distance between each individual in group II showed maximum inter-individual distance. The Nearest-neighbour distance was found to be the lowest in group I whereas there was a gradual increase in the distance in other groups. Group III and II represent the highest distance of 14 and 11. Zebrafish shoals have been found to disperse more widely in less disturbed conditions[31]. According to our findings, the zebrafish are not tightly clustered together, showing that they are stressed.
Social contact (fig. 5a and fig. 5b) between the fishes has also been markedly reduced in groups II and III a significant outcome of autism-like behaviour in zebrafish. As zebrafish explore social groups, they often tend to spend more time near one another, demonstrating kin recognition/preference during social research. Zebrafish spent the majority of their time swimming in dynamic groups in the shoaling test, which were distinguished by short inter-fish distances, lower zebrafish group area/diameters, and relative polarization. Reduced polarisation of fish shoals, looser and larger schools and a higher percentage of fish leaving the group and spending time outside the shoal might prove the disorganised social organisation in zebrafish[32].
All the social behaviours were carried out to understand the neuroprotective effects of chrysin against valproic acid-induced autism-like behaviour. Previous studies have shown that zebrafish exposed to valproic acid exhibit behavioural despair, which is associated with ASD-like traits and behavioural impairments like hyperactivity and social deficiency[33]. Chrysin has been proven to diminish dopamine depletion and protect dopaminergic neurons in the brain from neurodegeneration[34]. Treatment with chrysin has also proven to effectively improve dopamine levels in the hippocampus and prefrontal cortex of the brain[35]. Chrysin has been found to have a Gamma-Aminobutyric Acid (GABA) mimetic effect and to alter the GABAA receptor, hence reducing anxiety and depression-like behaviour[36].
In conclusion, our findings demonstrated that chrysin could counteract the behavioural changes in zebrafish animal models caused by valproic acid immersion and intraperitoneal injection. Furthermore, this research reveals intraperitoneal injection as a promising approach to induce Autism-like behaviour. No locomotor alterations were detected 24 h after the behavioural session. The therapeutic effect of chrysin can be attributed to a progressive influence on social behaviour. Although more research is needed to fully comprehend the neurochemical pathways underlying chrysin's effects on valproic acid-induced Autism-like behaviour, our study implements the use of zebrafish models as emerging tools to investigate the neuroprotective effects of diverse drugs.
Acknowledgements:
Authors are thankful to PSG College of Arts and Science for providing facilities and support to conduct the research.
Conflict of interest:
The authors declared no conflict of interests.
References
- Jiang M, Lu T, Yang K, Li X, Zhao L, Zhang D, et al. Autism spectrum disorder research: knowledge mapping of progress and focus between 2011 and 2022. Front Psychiatry 2023;14:1096769.
[Crossref] [Google Scholar] [PubMed]
- Hodges H, Fealko C, Soares N. Autism spectrum disorder: Definition, epidemiology, causes, and clinical evaluation. Transl Pediatr 2020;9(Suppl 1):S55-65.
[Crossref] [Google Scholar] [PubMed]
- Lord C, Cook EH, Leventhal BL, Amaral DG. Review autism spectrum disorders coordinating vocalizations with their intentions, and com. Neuron 2000;28:355-63.
- Saki K. Autism: Synthetic- and plant-derived control and treatment. 2018;12(3):483-9.
- Nabavi SF, Braidy N, Habtemariam S, Orhan IE, Daglia M, Manayi A, et al. Neuroprotective effects of chrysin: From chemistry to medicine. Neurochem Int 2015;90:224-31.
[Crossref] [Google Scholar] [PubMed]
- Naz S, Imran M, Rauf A, Orhan IE, Shariati MA, Shahbaz M, et al. Chrysin: Pharmacological and therapeutic properties. Life Sci 2019;235:116797.
[Crossref] [Google Scholar] [PubMed]
- Eissa N, Al-Houqani M, Sadeq A, Ojha SK, Sasse A, Sadek B. Current enlightenment about etiology and pharmacological treatment of autism spectrum disorder. Front Neurosci 2018;12:304.
[Crossref] [Google Scholar] [PubMed]
- Shams S, Rihel J, Ortiz JG, Gerlai R. The zebrafish as a promising tool for modeling human brain disorders: A review based upon an IBNS symposium. Neurosci Biobehav Rev 2018;85:176-190.
[Crossref] [Google Scholar] [PubMed]
- Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013;496(7446):498-503.
[Crossref] [Google Scholar] [PubMed]
- McCann LI, Koehn DJ, Kline NJ. The effects of body size and body markings on nonpolarized schooling behavior of zebra fish (Brachydanio rerio). J Psychol 1971;79(1):71-5.
[Crossref] [Google Scholar] [PubMed]
- Stewart A, Kadri F, DiLeo J, Min Chung K, Cachat J, Goodspeed J, et al. The developing utility of zebrafish in modeling neurobehavioral disorders. Int J Comp Psychol 2010;23(1):104-20.
- Norton W, Bally-Cuif L. Adult zebrafish as a model organism for behavioural genetics. BMC Neurosci 2010;11(1):1-11.
[Crossref] [Google Scholar] [PubMed]
- Kalueff AV, Stewart AM, Gerlai R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol Sci 2014;35(2):63-75.
[Crossref] [Google Scholar] [PubMed]
- Choo BK, Kundap UP, bin Johan Arief MF, Kumari Y, Yap JL, Wong CP, et al. Effect of newer Anti-Epileptic Drugs (AEDs) on the cognitive status in pentylenetetrazol induced seizures in a zebrafish model. Prog Neuro-Psychopharmacology Biol Psychiatry 2019;92:483-93.
[Crossref] [Google Scholar] [PubMed]
- German-Ponciano LJ, Costa BP, Feitosa LM, dos Santos Campos K, da Silva Chaves SN, Cueto-Escobedo J, et al. Chrysin, but not flavone backbone, decreases anxiety-like behavior in animal screens. Neurochem Int 2020;140:104850.
[Crossref] [Google Scholar] [PubMed]
- Franscescon F, Müller TE, Bertoncello KT, Rosemberg DB. Neuroprotective role of taurine on MK-801-induced memory impairment and hyperlocomotion in zebrafish. Neurochem Int 2020;135:104710.
[Crossref] [Google Scholar] [PubMed]
- Sison M, Gerlai R. Behavioral performance altering effects of MK-801 in zebrafish (Danio rerio). Behav Brain Res 2011;220(2):331-7.
[Crossref] [Google Scholar] [PubMed]
- Magno LD, Fontes A, Gonçalves BM, Gouveia Jr A. Pharmacological study of the light/dark preference test in zebrafish (Danio rerio): Waterborne administration. Pharmacol Biochem Behav 2015;135:169-76.
[Crossref] [Google Scholar] [PubMed]
- Maximino C, Marques de Brito T, Dias CA, Gouveia Jr A, Morato S. Scototaxis as anxiety-like behavior in fish. Nat Protoc 2010;5(2):209-16.
[Crossref] [Google Scholar] [PubMed]
- Pham M, Raymond J, Hester J, Kyzar E, Gaikwad S, Bruce I, et al. Assessing social behavior phenotypes in adult zebrafish: Shoaling, social preference, and mirror biting tests. Zebrafish Protocols for Neurobehavioral Research 2012:231-46.
- Joseph TP, Zhou F, Sai LY, Chen H, Lin SL, Schachner M. Duloxetine ameliorates valproic acid-induced hyperactivity, anxiety-like behavior, and social interaction deficits in zebrafish. Autism Res 2022;15(1):27-41.
[Crossref] [Google Scholar] [PubMed]
- Krishnan M, Kang SC. Vitexin inhibits acrylamide-induced neuroinflammation and improves behavioral changes in zebrafish larvae. Neurotoxicol Teratol. 2019;74:106811.
[Crossref] [Google Scholar] [PubMed]
- Conrad JL, Weinersmith KL, Brodin T, Saltz JB, Sih A. Behavioural syndromes in fishes: A review with implications for ecology and fisheries management. J Fish Biol 2011;78(2):395-435.
[Crossref] [Google Scholar] [PubMed]
- Cachat J, Stewart A, Grossman L, Gaikwad S, Kadri F, Chung KM, et al. Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nat Protoc 2010;5(11):1786-99.
[Crossref] [Google Scholar] [PubMed]
- Kalueff AV, Gebhardt M, Stewart AM, Cachat JM, Brimmer M, Chawla JS, et al. Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish 2013;10(1):70-86.
[Crossref] [Google Scholar] [PubMed]
- Angiulli E, Pagliara V, Cioni C, Frabetti F, Pizzetti F, Alleva E, et al. Increase in environmental temperature affects exploratory behaviour, anxiety and social preference in Danio rerio. Sci Rep 2020;10(1):1-12.
[Crossref] [Google Scholar] [PubMed]
- Blaser RE, Rosemberg DB. Measures of anxiety in zebrafish (Danio rerio): Dissociation of black/white preference and novel tank test. PLoS One 2012;7(5):e36931.
[Crossref] [Google Scholar] [PubMed]
- Gerlai R. Zebra fish: An uncharted behavior genetic model. Behav Genet 2003:33(5):461-8.
[Crossref] [Google Scholar] [PubMed]
- Stephenson JF, Whitlock KE, Partridge JC. Zebrafish preference for light or dark is dependent on ambient light levels and olfactory stimulation. Zebrafish 2011;8(1):17-22.
[Crossref] [Google Scholar] [PubMed]
- Stewart AM, Nguyen M, Wong K, Poudel MK, Kalueff AV. Developing zebrafish models of Autism Spectrum Disorder (ASD). Prog Neuro-Psychopharmacology Biol Psychiatry 2014;50:27-36.
[Crossref] [Google Scholar] [PubMed]
- Miller N, Gerlai R. Quantification of shoaling behaviour in zebrafish (Danio rerio). Behav Brain Res 2007;184(2):157-66.
[Crossref] [Google Scholar] [PubMed]
- Capurro V. New pharmacological tools for autism research: Oxytocin receptor mutant mice and zebrafish as neurobehavioural models. 2009.
- Chen J, Lei L, Tian L, Hou F, Roper C, Ge X, et al. Developmental and behavioral alterations in zebrafish embryonically exposed to Valproic Acid (VPA): An aquatic model for autism. Neurotoxicol Teratol 2018;66:8-16.
[Crossref] [Google Scholar] [PubMed]
- Del Fabbro L, Goes AR, Jesse CR, de Gomes MG, Souza LC, Ladd FV, et al. Chrysin protects against behavioral, cognitive and neurochemical alterations in a 6-hydroxydopamine model of Parkinson’s disease. Neurosci Lett 2019;706:158-63.
[Crossref] [Google Scholar] [PubMed]
- Bortolotto VC, Pinheiro FC, Araujo SM, Poetini MR, Bertolazi BS, de Paula MT, et al. Chrysin reverses the depressive-like behavior induced by hypothyroidism in female mice by regulating hippocampal serotonin and dopamine. Eur J Pharmacol 2018;822:78-84.
[Crossref] [Google Scholar] [PubMed]
- Rodríguez-Landa JF, Guillén-Ruiz G, Hernández-López F, Cueto-Escobedo J, Rivadeneyra-Domínguez E, Bernal-Morales B, et al. Chrysin reduces anxiety-like behavior through actions on GABAA receptors during metestrus-diestrus in the rat. Behav Brain Res 2021;397:112952.
[Crossref] [Google Scholar] [PubMed]