- *Corresponding Author:
- Xiaohui Guan
Department of Gastroenterology
Affiliated Hospital of BeiHua University
Jilin 132012
PR China
E-mail: 857173680@qq.com
| Date of Received | 27 October 2020 |
| Date of Revision | 14 February 2021 |
| Date of Acceptance | 29 March 2021 |
| Indian J Pharm Sci 2021;83(2):337-344 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Gastric cancer is the most common malignant tumor disease in the human digestive system. It ranks first in the incidence of cancer-related diseases in China. It is a serious threat to the health of Chinese people. Vitamin E Succinate is a natural Vitamin E derivative and has been confirmed to have an inhibitory effect on tumor cell growth. Based on this, the article explores the effect of Vitamin E Succinate on inhibiting the growth of human gastric cancer cells and aims to provide a reference for the clinical diagnosis and treatment of gastric cancer. This article first systematically introduces Vitamin E Succinate and the related theoretical knowledge of gastric cancer and lays a sufficient theoretical foundation for the following research on Vitamin E Succinate inhibiting the growth of human gastric cancer cells and inducing apoptosis of gastric cancer cells. The human gastric cancer cell specimens and Vitamin E Succinate solution were formulated and the effect of Vitamin E Succinate on the growth of human gastric cancer cells was tested. According to the concentration of Vitamin E Succinate, the gastric cancer cells in the experiment were divided into Vitamin E Succinate 10 mg/ml dose group, Vitamin E Succinate 10 mg/ml dose group, Vitamin E Succinate 15 mg/ml dose group and the blank control group, the growth curve of gastric cancer cells, cell colony determination, cell division index determination and cell 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide detection and analysis were used to compare the changes in cell growth between different dose groups; the final result is that Vitamin E Succinate has a significant inhibitory effect on the growth of gastric cancer cells (p<0.05)and as the dose concentration increases and the treatment time increases, its inhibitory effect on the cells is more significant.
Keywords
Vitamin E Succinate, human gastric cancer cells, promote apoptosis, inhibitory effect
Gastric cancer is the fourth type of high-incidence tumor after lung cancer, breast cancer and colon cancer. Its incidence rate ranks first among cancer-related deaths and its lethality rate is the highest. Cancerrelated deaths are the second place and are one of the most important killers that threaten human health. Our country is an area with a high incidence of gastric cancer and the number of deaths from gastric cancer each year accounts for a quarter of the deaths from malignant tumors. At present, the most important way to treat gastric cancer is surgical treatment. Although the diagnosis and treatment level of gastric cancer has improved to a certain extent in recent years, the detection rate of gastric cancer is caused by the asymptomatic early stage of gastric cancer and the unclear mechanism and causes of the disease. And the radical cure rate is not high and the prognosis effect is not ideal.
Gastric cancer is the result of a variety of factors such as family genetics, living environment and living habits. Due to its complicated pathological mechanism, the exact cause of the disease is not yet clear. However, many studies have found that Vitamin E Succinate (VES) can inhibit the growth of tumor cells and promote tumor cell apoptosis. VES is a natural Vitamin E derivative. It can inhibit a variety of tumor cells without any negative effects on the growth of other normal cells in the human body. Therefore, it is often used as a tumor chemopreventive and therapeutic agent. The purpose of this article is to study the inhibitory effect of VES on human gastric cancer cells, hoping to provide reference and reference for the clinical treatment of gastric cancer patients in our countryand to contribute to reducing the prevalence of gastric cancer in our country and promoting national health.
Because gastric cancer poses a fatal threat to human health, many experts and scholars in the medical field have started research on it early in order to prevent and treat it in time. Xianmin Mu and Ting Zhao pointed out that it is now generally accepted that altered cell metabolism is a sign of cancer cells and the resulting abnormal metabolites can cause metabolic and nonmetabolic disordersand may transform into malignant tumors[1]. In addition, Yu, Yang and Hou Liying also conducted research on VES. They showed that VES is a derivative of Vitamin E and is a promising cancer chemopreventive agent. It inhibits tumor growth by inducing apoptotic cell death. They pointed out that the effect of VES on autophagy is still unclear. Autophagy is a complex programmed process that helps cells survive under certain stress conditions by degrading certain cytoplasmic substances[2]. In short, there are many studies on VES inhibiting the growth of gastric cancer tumor cells. This article only puts forward some superficial views and insights on the basis of previous studies, hoping to provide some help for the clinical treatment of gastric cancer.
The innovations of this article are mainly reflected in the following aspects: Gastric cancer, as one of the main malignant tumors threatening human health, has a very high prevalence and fatality rate. There are many deaths due to gastric cancer each year. Based on this, the content of this article has great social practical significance and discussion value; VES is a natural derivative of Vitamin E, which has an inhibitory effect on the formation and growth of a variety of tumor cells. This article compares the effects of VES and a-tocopherol on gastric cancer cells to explore the inhibitory effect of VES on the growth of gastric cancer cells.
Materials and Methods
Vitamin E
Vitamin E is a fat-soluble vitamin, also known as Vitamin E, tocopheroland pregnancy-producing phenol. It has strong antioxidant properties and is one of the most important antioxidants. It is often used in skin care products such as creams and lotions. In addition, many health products and nutrition products also contain Vitamin E, which is an antioxidant and nutrient with good effects. Its appearance is a kind of gold Yellow or light yellow oil, with a mild special odor, it is easily oxidized under light and presents a dark red color. Vitamin E was first discovered in the 1920s. At first it was just a simple fat-soluble dietary factor. Later, after continuous research and exploration by people, it was successfully separated into crystals. This crystal was artificially synthesized into today’s vitamins E[3-4].
Vitamin E is the general term for tocopherols. According to the side chain structure, it can be divided into tocopherols and tocotrienols. According to the position and number of methyl groups, they are divided into 4 types of fat-soluble vitamins, namely α, Β, γ, δ tocopherols and α, β, γ, δ tocotrienols, of which α tocopherol has the highest content and the best physiological activity. The chemical structure of tocopherol is extremely unstable and is easily oxidized to oxidized tocopherol and tocopherol hydroquinone. The tocopherol-related compound has a certain activity when the substituents are different, but the activity of tocopherol will obviously decrease[5].
Vitamin E has many biological functions, can prevent and cure certain diseases and has positive effects and effects on anti-oxidation, anti-aging, nutrition and beauty, etc., which are embodied in the following aspects: First, anti-oxidation, slow down agingand reduce the production of wrinkles; Second, reduce the oxygen consumption of cells, enhance the endurance of muscles and bonesand prevent leg cramps and stiffness of hands and feet; Third, improve body fat metabolism, prevent the occurrence of hypertension, coronary heart disease and atherosclerosis, improve human blood lipid abnormalitiesand lower cholesterol content; Fourth, anti-radiation, can effectively protect the skin from ultraviolet rays, reduce scars and pigment deposition; Fifth, promote blood circulation, prevent cell pathology and inhibit the growth of cancer cells; Sixth, it helps prevent inflammatory skin diseases, regulate the use of acid in the human body and protect the skin and mucous membranes; Seventh, it can improve the blood microcirculation of the scalp hair follicles, ensure the nutrient supply of the scalp, prevent hair loss and promote hair growth; eighth, promoting the secretion of sex hormones is helpful for women to improve fertility and prevent miscarriage[6,7].
VES is a derivative of natural Vitamin E. It is a compound formed by esterification of succinic acid with the 6-hydroxyl of α-tocopherol. Studies have shown that Vitamin E has an antioxidant effect after esterification with succinic acid, which can inhibit the growth and regeneration of a variety of tumor cells, such as leukemia cells, breast cancer cells, lymphoma cellsand gastric cancer cells.
Gastric cancer
Gastric cancer is a type of cancer disease with extremely high morbidity and mortality among various malignant tumor diseases. Gastric cancer ranks first in the incidence of cancer-related diseases in our country and has obvious geographical and gender differences. The prevalence of gastric cancer in the eastern coastal cities and northwestern regions of our country is significantly higher than that in the southern regions. The patients are mostly men and most of them are middle-aged and elderly people over 50 y old. However, in recent years due to changes in dietary structure and the pressure of life and work, the age of onset of gastric cancer has shown a younger phenomenon and more and more young people are also prone to gastric cancer.
Gastric cancer is a malignant tumor that occurs in the epithelium of the gastric mucosa. Medical experts say that gastric cancer may occur in any part of the stomach. In most cases, it occurs in the antrum, greater curvature and lesser curvature of the stomach and the front and back walls. The vast majority of patients with gastric cancer cannot find any obvious symptoms at an early stage and only show symptoms similar to general gastritis, gastric ulcers and other chronic gastric diseases. Therefore, they are often overlooked, delaying the diagnosis period and delaying early treatment, leading to gastric cancer. The mortality rate of patients is extremely high. In addition, the prognosis of gastric cancer and the success rate of surgical treatment are closely related to its pathological staging and grading. However, most pathology has a longer diagnosis period because of no obvious symptoms in the early stage. Once diagnosed, it is likely that the disease has developed into the middle and late stages. At this time, even with barely treatment, the possibility of postoperative recurrence is still very high[8].
First: Affected by regional environment and eating habits. The incidence of gastric cancer in our country has distinct geographical differences. The incidence of gastric cancer in the northwest and eastern coastal areas is much higher than that in the south, the incidence of gastric cancer in the group with long-term lifestyle and irregular diet is significantly higher than that in the group with a healthy lifestyle. And the prevalence of gastric cancer in people who often consume smoked and pickled foods is also higher. This is related to the excessive carcinogens or pre-carcinogens such as nitrite in the food. In addition, smokers suffer more than nonsmokers. The chance of stomach cancer is also high.
Second: It is related to Helicobacter pylori infection. Data show that in the high-incidence area of gastric cancer in our country, more than 60 % of adults are infected with Helicobacter pylori and once infected with Helicobacter pylori, it is likely to develop into gastric cancer. Helicobacter pylorus is a kind of bacteria that is prone to carcinogenesis. It can cause chronic inflammation, leading to errors in DNA replication and free radical formation in cell proliferation. It can easily cause gastritis, peptic ulcer and lymphoproliferative gastric lymphoma. The human stomach hurts greatly.
Third: precancerous lesions derived from gastric disease. Gastric polyps, gastritis, gastric ulcersand the remnant stomach after partial gastric resection are all prone to cause gastric cell mucosal lesionsand these lesions may be accompanied by varying degrees of chronic inflammation and cell proliferation, which will occur in severe cases and directly into cancer. Precancerous lesions refer to the borderline pathological changes in the process of gastric cell mucosal epithelial tissue transforming into cancer.
Fourth: It is related to genetic inheritance. Studies have found that members of the family with a history of gastric cancer tend to have a higher incidence of gastric cancer. The pathology of gastric cancer is a complex and changeable process involving oncogenes, tumor suppressor genes, apoptosisand metastatic genes. In addition, there are various forms of alteration of the gene itself[9].
First, it is divided into early gastric cancer and advanced gastric cancer according to the general shape. Early gastric cancer refers to the cancer cells distributed in the gastric mucosa and its lower layer, which can be divided into protruding, flatand depressed types in a visible form; advanced gastric cancer refers to cancer cells that have penetrated into the stomach and exceeded the surface of the gastric mucosa. It is divided into four types: polyp type, local ulcer type, infiltrating ulcer type and diffuse infiltrating type.
Second, according to the classification of pathological mechanisms, it can be divided into adenocarcinoma, adenosquamous cell carcinoma, squamous cell carcinoma and carcinoid. Most gastric cancers belong to gastric adenocarcinoma.
Third, according to the location of the disease, it can be divided into gastric antrum cancer and gastric body cancer[10]. At present, the main method of clinical treatment of gastric cancer is surgical treatment, with other supplementary chemotherapy and rehabilitation nursing. Specifically, there are the following:
The first type: surgical treatment. Including radical surgical treatment and palliative surgical treatment. Radical surgery is generally used for patients with advanced gastric cancer. The stomach has been completely or partially cancerous but is about to infiltrate other parts. In principle, the stomach including cancerous tissue must be cut off. All or part of the lymph nodes around the stomach tissue are cleared and then the artificial digestive tract is reconstructed by medical technology; palliative surgery is used for gastric cancer patients with unresectable cancer, in order to relieve symptoms caused by perforation, bleeding and other complications. One kind of surgery is a conservative treatment. This kind of surgery includes jejunos to our and perforation repair.
The second type: chemotherapy. Chemotherapy is an adjuvant treatment used before, during and after surgery for gastric cancer patients, mainly for repairing and prolonging survival. In general, many patients with advanced gastric cancer will choose chemotherapy in order to relieve pain, delay the growth of tumors, improve their symptoms and prolong their lives. It has certain short-term effects.
The third type: targeted therapy. Targeted therapy is mainly to suppress specific cancer cells with drugs, damage cancer cellsand promote cancer cell apoptosis. Such drugs mainly include epidermal growth factor inhibitors, apoptosis promoters and cell cycle inhibitors and so on.
The fourth type: Supportive treatment. This treatment method aims to alleviate the suffering of patients, improve the quality of life of patients, help patients relieve their psychological burden, improve their appetite, etc.[11].
Mechanism of vitamin e succinate inhibiting the growth of gastric cancer cells
VES inhibits DNA synthesis of gastric cancer cells. Cell proliferation can be achieved through cell cycle changes and cell proliferation and growth can be inhibited once the cell cycle stops. Some experimental studies have shown that the intake inhibition rate of gastric cancer cells after 48 h of treatment with 5 mg/L VES and 10 mg/L VES at 24 and 48 h is compared with that of normal controls. There is a big difference between the groups and the intake dose has a great relationship with the length of time. By comparing the intake of cancer cell inhibitors in different periods, it is found that the longer the time, the better the effect of VES on inhibiting the proliferation of gastric cancer cells.
At present, studies on VES-induced tumor cells are mainly focused on signal pathways and signal factors. Among them, transforming growth factor is the most important factor that regulates cell growth, differentiation and death and transforming growth factor beta (TGF-β) as an epithelial proliferation inhibitor is effective. The growth and proliferation of these cancer cells and tumors have significant inhibitory effects and effects. VES is an excellent cell regulator, it can prevent gastric cancer cell DNA synthesis to prevent its growth and induce its apoptosis[12].
Effect of vitamin e succinate on the growth of human gastric cancer cells
In order to more specifically analyze and explore the mechanism of VES inhibiting human gastric cancer cellsand to verify its positive effect on the clinical diagnosis and treatment of gastric cancer, this article cultured and developed human gastric cancer cell specimens to conduct VES inhibition of human gastric cancer research experiments on cell growth.
The VES, α-tocopherol, Roswell Park Memorial Institute (RPMI) 1640 medium and tritiated thymidine (H-TdR) used in this experiment were all purchased from a regular biopharmaceutical company. The culture of human gastric cancer cells and the preparation of the VES solution are as follows:
The original specimens of human gastric cancer cells were purchased from the Provincial Cancer Institute. First prepare the culture medium of gastric cancer cells, mix 15 % bovine serum, 100 IU/ml penicillin, 100 μg/ ml streptomycin and 3 mmol/L L-glutamine into the RPMI-1640 culture mediumand then. The original gastric cancer cell specimens are placed in it and placed in an incubator with a temperature of 37° and 4 % carbon dioxide for routine culture.
Firstly, VES was formulated into a stock solution with a concentration of 10 mg/ml with absolute ethanoland then diluted into VES solutions with different concentrations, namely 5 μg/ml, 10 μg/ml using RPMI- 1640, 15 μg/ml and finally add absolute ethanol to these VES solutions with different concentrations, so that the concentration of ethanol in the solution becomes 1 ml/l.
The equipment used in the experiment are: fluorescent inverted microscope, cytometer, electronic balance, incubator, detector, etc.; reagents: VES, RPMI-1640 culture medium, bovine serum, penicillin, streptomycin, L-Glutamine, ethanol, etc.
The experiment mainly involves the determination of cell growth curve, colony, division index and MTT detection. The specific steps are as follows:
Stomach cancer cells were inoculated into a 24-well plate at a density of 1×1010 per well and then placed in an incubator containing carbon dioxide for 24 h. After that, the incubator was replaced with two different culture media, that is, the first is RPMI-1640 culture medium mixed with 1ml/L of absolute ethanol and 15 % newborn calf serum; the second is a VES solution with concentrations of 5 %, 10 % and 15 % respectively. After the culture medium was changed, the culture was continued for another week, during which the medium was changed once a day and the cells in the two different culture mediums were digested 4 wells each.
Stomach cancer cells were seeded in a 24-well plate at a density of 250 cells per well and then placed in an incubator containing carbon dioxide for 24 h. Afterwards, the culture medium in the incubator was replaced with a blank control solution and a VES solution with a concentration of 5 %, 10 % and 15 % and cultured for another 24-48 h. Then the culture medium was replaced with RPMI-1640 culture medium mixed with 1ml/L absolute ethanol and 15 % newborn calf serum and then cultured for about 10 d. After the incubation period, wash the gastric cancer cells with buffer solution, fix them with methanol, stain them with staining solutionand then count the number of cell colonies in each well in the 24-well plate. Only those with more than 50 cells are counted as a colony, the formula of colony formation rate is: colony formation rate= (average number of colonies/number of single cells inoculated) ×100 %.
Prepare a 6-well culture plate containing a cover glass, inoculate the gastric cancer cells into the culture plate at a density of 1×1010 per well and then routinely cultivate for 24 h, then replace the culture medium with a blank control solution and a concentration of 5 %, 10% and 15 % VES solution, continue to incubate for 24-48 h, after the end, wash the cells with buffer solution, fix with methanol and stain with staining solution and then observe the cells under a microscope. The number of dividing cells and the calculation formula of the cell division index are: division index= (number of dividing cells/total number of cells) ×100 %.
As mentioned above, general Vitamin E and tocopherol have no inhibitory effect on the growth of cancer cellsand the esterification of tocopherol and succinic acid has the function of inhibiting the growth of tumors and cancer cells. In order to further test its truth, we compared the effects of VES and α-tocopherol on gastric cancer cells by MTT method. First, inoculate gastric cancer cells in a culture plate at a density of 500 cells per well. After regular culture for 24 h, replace the culture medium with a blank control solution and VES with a concentration of 5 %, 10 % and 15 %. Solution and RPMI-1640 culture medium mixed with 1 ml/l absolute ethanol and 15 % newborn calf serum and then continue to culture for 1 w. Take 5 wells of cells from each group of culture medium, add MTT with a concentration of 4 mg/ml into it, suck out the culture medium with a pipette after 2 h, add 0.1ml DMSO, then shake welland finally measure the light with a cytometer. Density and calculate the survival rate of cells by comparison with the control group, the formula is: cell survival rate= (experimental group optical density/control group optical density) ×100 %.
This experiment aims to explore how VES inhibits the growth and induces apoptosis of gastric cancer cells by inhibiting DNA synthesis. Therefore, the main indicators tested in the experiment are cell growth curve, cell colony formation rate and cell growth. Division index and MTT detection of cells.
In addition to the above formula, this experiment also used the data statistical analysis software SPSS22.0 in the analysis and comparison of the experimental resultsand used variance and t-test to test the accuracy of the experimental results. p<0.05 represents the difference. Significantly statistically significant, p<0.01 means that the difference is extremely statistically significant, p>0.05 means that the difference is not statistically significantand the mathematical formula of variance and t-test is as follows:
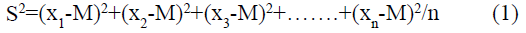

Results and Discussion
According to the third part of the experiment of VES inhibiting the growth of human gastric cancer cells, we divided the observed cells into three groups according to different doses of culture fluid, namely the blank control group, VES 5 mg/ml, VES 10 mg/ml and VES 15 mg/ml to test the inhibitory effect of VES on gastric cancer cells by observing and comparing the changes of gastric cancer cells between different dose groups. In this chapter, we will analyze and discuss the experimental results in detail.
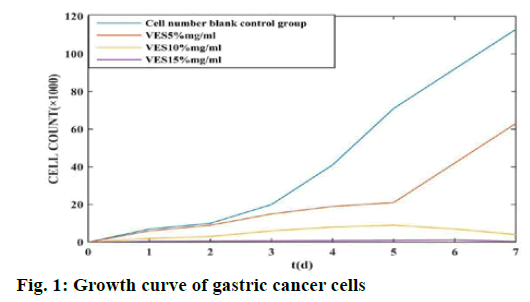
In the experiment, the blank control group and each dose group of VES were placed in a culture plate for continuous culture for 1 w and the number of living cells in the culture plate was calculated. The results are shown in fig. 1.
According to fig. 1, the gastric cancer cells in the blank control group began to grow rapidly on the 2nd d after inoculation. After 1 w of culture, the number of cells grown exceeded 100×1000; while in the VES dose group, in the VES 5 mg/ml group, the number of live cells increased slowly due to the inhibitory effect of VES in the first 5 dand the live cells began to increase sharply after the 5th d; in the VES 10 mg/ml group, the number of live cells increased slowly. The number of cells is smaller than that of the VES 5 % mg/ml dose groupand the number of viable cells still has a downward trend by the 5th d; the VES 15 mg/ml group has the least number of live cells among all dose groupsand its cell number has been controlled at 1000. This shows that VES has a significant inhibitory effect on the growth of gastric cancer cells and the greater its concentration, the more significant the inhibitory effect on cancer cells.
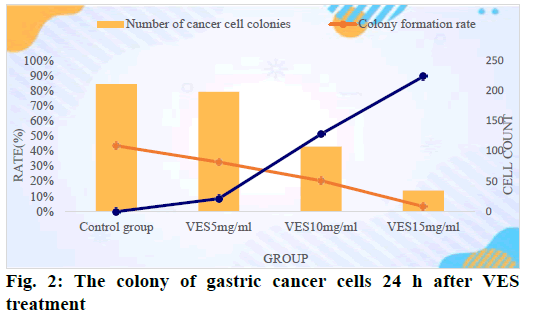
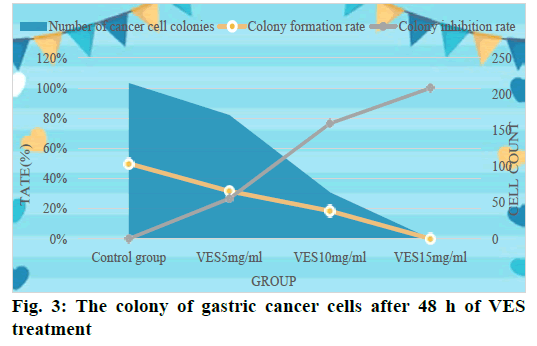
Table 1 shows the colony formation of gastric cancer cells in the experiment. According to Table 1, VES in different dose groups has different degrees of inhibition on the colony formation of gastric cancer cellsand with the dose of VES and the gradual increase in the concentration and the prolongation of the processing time of cells, the more obvious its inhibitory effect on cancer cells. When the VES dosage reached 15 mg/ml and after 48 h of treatment, the colony formation rate became 0 and the inhibition rate reached 100 %.
| VES dose (μg/ml) | Number of cancer cell colonies | Colony formation rate (%) | Colony inhibition rate (%) | |||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |
| 0 | 210.9±24.36 | 215.2±17.27 | 43.5 | 49.9 | 0 | 0 |
| 5 | 198.1±13.47 | 171.0±11.43 | 32.7 | 31.6 | 8.6 | 26.7 |
| 10 | 107.5±9.75 | 64.13±8.76 | 20.4 | 18.5 | 51.3 | 76.5 |
| 15 | 34.9±4.60 | 0 | 3.4 | 0 | 89.4 | 100 |
Table 1: Colony Status of Gastric Cancer Cells in Each Dose Group
In order to observe the colonization of gastric cancer cells in each dose group more intuitively and to analyze the inhibitory effect of VES on gastric cancer cells, we plotted the data in the table as shown in fig. 2. According to the experimental results, we counted the cell division of each group as shown in fig. 3.
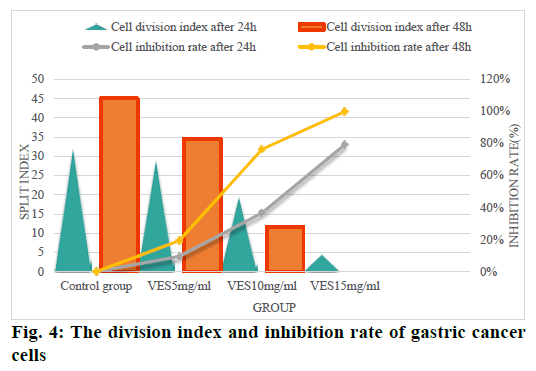
From fig. 4, we can see that after gastric cancer cells were treated with unequal doses of VES, their cell division was affected to varying degrees according to the length of the treatment time. Among them, the VES 15 mg/ml dose group after 48 h of treatment, the cell division index became 0 and the inhibition rate reached 100 %; the cell division index of the VES 10 mg/ml dose group was significantly lower 48 h after treatment than 24 h after treatment. There is a significant statistical difference (p<0.05) and the cell growth inhibition rate increased sharply to more than 70 % after 48 h of treatment; although the cells in the VES 5 % mg/ ml dose group were also inhibited to a certain extent, the effect was far inferior VES 15 mg/ml and VES 10 mg/ml dose groups; while in the control group, the cell division index gradually increased with the increase of time without any inhibitory effect. This shows that as the content of VES increases, its inhibitory effect on the growth of gastric cancer cells gradually increasesand the inhibition rate is also increasing.
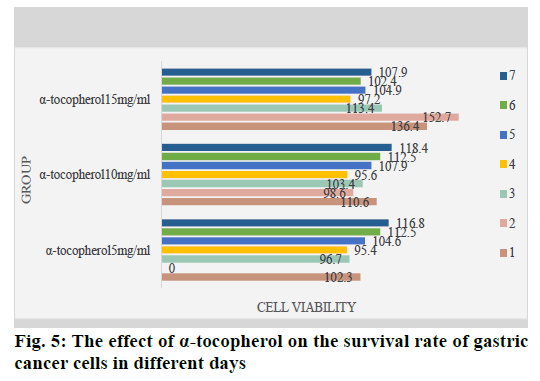
Through the MTT detection of VES and α-tocopherol, we found that in the treatment of gastric cancer cells with different concentrations of VES and α-tocopherol, the gastric cancer cells of each dose group were affected differently. Among them, the cytostatic effect of the VES 10 mg/ml dose group and the VES 15 mg/ ml dose group was the most obvious (p<0.05), which was completely inhibited after 1 w of treatment; while the same dose of α-tocopherol had no effect on gastric cancer cells. Play any inhibitory effect, but promote the growth of cancer cells to a certain extent. The effect of α-tocopherol on the growth of gastric cancer cells is shown in fig. 5. This once again shows that Vitamin E itself has no inhibitory effect on cells and it has the function of inhibiting the growth of tumors and cancer cells by combining with succinate.
It can be seen from fig. 5 that after 1 w of treatment, the cell survival rate of each dose group did not change significantly, which indicates that α-tocopherol has no inhibitory effect on the growth of gastric cancer cells.
As the number one public enemy threatening human health, gastric cancer has a very high incidence and fatality rate. Because it has no obvious symptoms in the early stage of onset, it is often difficult to detect and effectively treat it in time. In most cases, patients diagnosed with gastric cancer generally have advanced to the advanced stage. For this reason, it is very important to find an active and effective way to diagnose and treat gastric cancer and inhibit the growth of tumors and cancer cells. VES is a natural Vitamin E derivative, which is formed by the esterification of α-tocopherol and succinate. Studies have found that general Vitamin E and α-tocopherol do not have the function of inhibiting the growth of cancer cells, but through combined with succinate esterification, it can effectively inhibit the growth of tumors and cancer cellsand promote cell apoptosis, which has a very positive effect on the diagnosis and treatment of gastric cancer in clinical medicine.
In this study, the effect of VES on the growth of gastric cancer cells confirmed its inhibitory effect on the growth of gastric cancer cells. It was also found that the greater the concentration of VES, the more obvious the inhibitory effect on gastric cancer cells and the better the effect.
References
- Mu X, Zhao T, Xu C, Shi W, Geng B, Shen J, et al. Oncometabolite succinate to promote angiogenesis by upregulating VEGF expression through GPR91-mediated STAT3 and ERK activation. J Clin Oncol 2017;35(15):e23000.
- Yu Y, Hou L, Song H, Xu P, Sun Y, Wu K. Akt/AMPK/mTOR pathway was involved in the autophagy induced by vitamin E succinate in human gastric cancer SGC-7901 cells. Mol Cell Biochem 2017;424(1):173-83.
- Deng W, Wang J, Zhang J, Cai J, Bai Z, Zhang Z. TET2 regulates LncRNA‐ANRIL expression and inhibits the growth of human gastric cancer cells. IUBMB life 2016;68(5):355-64.
- Zhao JG, Zhang L, Xiang XJ, Yu F, Ye WL, Wu DP, et al. Amarogentin secoiridoid inhibits in vivo cancer cell growth in xenograft mice model and induces apoptosis in human gastric cancer cells (SNU-16) through G2/M cell cycle arrest and PI3K/Akt signalling pathway. J BUON 2016;21(3):609-17.
- Hahm SW, Park J, Park KY, Son YS, Han H. Extracts of Opuntia humifusa fruits inhibits the growth of AGS human gastric adenocarcinoma cells. Prevent Nutr Food Sci 2016;21(1):31-7.
- Wu Y, Zhang J, Zheng Y, Ma C, Liu XE, Sun X. miR-216a-3p inhibits the proliferation, migration and invasion of human gastric cancer cells via targeting RUNX1 and activating the NF-κB signaling pathway. Oncol Resh 2018;26(1):157-71.
- Wu H, Liu S, Gong J, Liu J, Zhang Q, Leng X, et al. VCPA, a novel synthetic derivative of α-tocopheryl succinate, sensitizes human gastric cancer to doxorubicin-induced apoptosis via ROS-dependent mitochondrial dysfunction. Cancer lett 2017;393:22-32.
- Rahva M, Kerstetter J. Cutaneous Manifestation of Gastrointestinal Disease. J Gastrointest Oncol 2016;7(1):S44-54.
- Baskin Y, Kocal GC, Kucukzeybek BB, Akbarpour M, Kayacik N, Sagol O, et al. PDGFRA and KIT mutation status and its association with Clinicopathological properties, including DOG1. Oncol Res 2016;24(1):41-53.
- Li Y, Hong J, Li H, Qi X, Guo Y, Han M, et al. Genkwanin nanosuspensions: a novel and potential antitumor drug in breast carcinoma therapy. Drug Deliv 2017;24(1):1491-500.
- Xia Y, Weng B, Wang Z, Kang Y, Shi L, Huang G, et al. W346 inhibits cell growth, invasion, induces cycle arrest and potentiates apoptosis in human gastric cancer cells in vitro through the NF-κB signaling pathway. Tumor Biol 2016;37(4):4791-801.
- Chen XD, Hua XY, Kong XM, Wang XL. Sophoridine inhibits the proliferation of human gastric cancer MKN45 cells and promotes apoptosis. Acta Physiol 2018;70(4):391-6.