- *Corresponding Author:
- G. Jagtap
Department of Pharmacology, Bombay College of Pharmacy, Kalina, Santacruz (East), Mumbai-400 098, India
E-mail: jagtaparti@gmail.com
| Date of Submission | 28 February 2011 |
| Date of Revision | 31 August 2012 |
| Date of Acceptance | 3 September 2012 |
| Indian J Pharm Sci, 2012, 74 (5): 387-396 |
Abstract
Various studies have indicated that peptic ulcers occurring during the course of diabetic state are more severe and often associated with complications such as gastrointestinal bleeding. This study is the first attempt to understand the pathogenesis of gastric ulcers occurring during the diabetic state considering alternate biochemical pathways using suitable markers and its amelioration by Cuminum cyminum. In this study, diabetic rats showed a progressive increase in the stomach advanced glycated end products formation, gastric mucosal tumour necrosis factorâ??α and Thiobarbituric acid reactive substances levels as compared to normal control (nondiabetic) rats. There was decrease in gastric mucosal content, antioxidant enzymes and cellular ATPase enzyme levels of diabetic gastric mucosa when compared to the normal control group. mRNA expression of epidermal growth factor was found to be significantly higher as compared to normal control animals. Further methanol extract of Cuminum cyminum treatment to diabetic animals caused a reduction in blood glucose, and ulcer score when compared to diabetic control rats. It significantly increased gastric mucus content, antioxidant status and cellular ATPase enzyme levels as compared to diabetic control animals. Methanol extract of Cuminum cyminum inhibited advanced glycated end products formation in vitro as well as in vivo.
Keywords
Advanced glycated end products, Cuminum cyminum, diabetes, epidermal growth factor, gastric ulcer, gastric mucosal blood flow, tumour necrosis factor
Diabetes mellitus is a heterogeneous metabolic disorder characterised by hyperglycemia resulting from defective insulin secretion, resistance to insulin action or both. The evolution of numerous long‑term complications of diabetes mellitus correlates with the severity and duration of hyperglycemia [1]. Hyperglycemia is considered as the major cause of diabetic complications. Different pathways viz polyol pathway, modification of protein kinase C (PKC) activity, formation of advanced glycated end products (AGE) and oxidative stress have been reported that can result in diabetic complications [2].
Majority of the diabetic patients suffer from diabetic nephropathy, retinopathy, cardiopathy, neuropathy, however, little attention has been paid to the incidence and healing rate of peptic ulcer in diabetes because peptic ulcers among diabetics are considered infrequent. Recent experimental studies have shown that patients with long‑standing diabetes develop a variety of gastrointestinal symptoms such as abdominal pain, vomiting, diarrhoea, constipation and delayed gastric emptying [3]. Studies have indicated that peptic ulcers occurring in the course of diabetic state are more severe and often associated with complications such as gastrointestinal bleeding. Very few animal studies have been done to understand the pathogenesis of gastric ulcer during diabetes. Some studies show that the increased susceptibility to ulceration could be due to back diffusion of hydrogen ions in the stomach of diabetic rats, which played an important role in the formation of acute hemorrhagic ulcers [4]. While others have suggested the role of gastric mucosal blood flow (GMBF) and rapid changes in blood‑glucose levels [5]. There are reports of role of free radicals and loss of mucosal glycoprotein as one of the reasons for gastric ulceration in the course of diabetes [6]. Hence, the mechanism by which gastric ulcers are produced remains unclear. This is the first attempt to understand the pathogenesis of gastric ulcer considering various biochemical pathways leading diabetic complications.
Four major biochemical pathways studied which leads to diabetes related complications are polyol pathway; the hexosamine pathway; excess/inappropriate activation of PKC isoforms and accumulation of advanced glycation end products. While each pathway may be injurious alone, collectively they cause an imbalance in the mitochondrial redox state of the cell and lead to excess formation of reactive oxygen species (ROS). Increased oxidative stress within the cell leads to activation of the poly (ADP‑ribose) polymerase (PARP) pathway, which regulates the expression of genes involved in promoting inflammatory reactions.
Traditional system of medicine, Ayurved mentions the use of plants for acute as well as chronic ailments, which are commonly used in India. Cuminum cyminum Linn., commonly known as Jeerak, is consumed in large quantities by Indians. Literature indicates the evidence of hypoglycemic effect of C. cyminum [7,8]. Most recently cuminaldehyde derived from C. cyminum seed oil showed aldose reductase (AR) and alpha glucosidase inhibitory activity in vitro [9]. This study was undertaken to evaluate the effect of Jeerak as an antihyperglycemic and to explore its role in the modulation of diabetic complications in vivo.
Materials and Methods
Streptozotocin (STZ) was obtained from Sisco Research Laboratories, India. A diagnostic kit for the estimation of glucose was obtained from Erba Diagnostics, Germany. All the other chemicals used were of analytical grade. Adult Sprague Dawley rats (190‑210 g) of either sex were housed in standard polypropylene cages with wire mesh top and maintained at 23±2° with 12‑h light–dark cycle. Animals were fed with standard pellet diet and water ad libitum. Study protocols were approved by Institutional Animal Ethics Committee and experiments were performed in accordance with Committee for the Purpose of Control and Supervision of Experiments on Animals (India) (CPCSEA) guidelines.
Extract preparation and toxicity study
Seeds of C. cyminum were authenticated by an ayurvedic clinician in Mumbai. Seeds were extracted with methanol in a soxhlet assembly for 48 h. The methanol extract of Cuminum cyminum (MCC) was concentrated to obtain a semisolid mass of 10‑12% w/w; stored at 4‑8°. Acute oral toxicity study was performed as per Organisation for Economic Co-operation and Development guideline 423 (OECD 423).
Phytochemistry
Qualitative phytochemical evaluation was performed as described in Khandelwal [10]. Phenol and flavonoid content of MCC (0.2 mg/ml) was estimated by Folin–Ciocalteu reagent [11] and aluminium chloride (AlCl3) [12], respectively. High performance thin layer chromatography (HPTLC) analysis of MCC was performed using Camag Scanner III with WINCATS (Ver‑1.1.3) data processor on precoated silica gel 60 F254 thin layer chromatography plates using toluene, ethyl acetate, methanol, distilled water and glacial acetic acid (3:3:3:1:1) as solvent system [13]. Peaks were observed in UV/Vis, photodocumented and respective Rf values were noted.
In vitro radical scavenging assay
Solution of MCC (10 mg/ml) was prepared in dimethyl sulphoxide. Ability of the extract to scavenge superoxides generated in a nonenzymatic phenazine methosulfate–nicotinamide adenine dinucleotide (PMS–NADH) system [14] was studied at different concentrations (10‑1600 μg/ml). Ability to scavenge 1,1‑diphenyl‑2‑picrylhydrazyl (DPPH) free radical [15] was studied in MCC (1 mg/ml) prepared in 95% ethanol at various concentrations (10‑320 μg/ml). Concentration required for 50% inhibition of formation of superoxides and loss of DPPH colour was determined graphically.
In vitro bovine serum albumin glycation study
D‑Glucose (200 mM) was incubated with bovine serum albumin (BSA) (10 mg/ml) in phosphate buffer (50 mM, pH 7.4) containing 0.02% (w/v) sodium azide and different concentrations of MCC (200‑1400 μg/ml) at 37° for 7 days [16]. Fluorescence was measured at an excitation/emission wavelength of 370/440 nm and 335/385 nm to quantify AGE and pentosidine compounds, respectively.
Effect of MCC on serum glucose in oral glucose tolerance test
Normal Sprague Dawley rats were divided into four groups of six animals each. The animals were fasted overnight for 14 h. MCC was administered to three groups of rats at a dose of 100, 200, 400 mg/kg 1 h before the glucose load. Vehicle control rats received 1% sodium carboxymethyl cellulose suspension. Blood was collected just before the glucose load (0 h). Glucose load comprised of 50% glucose solution administered at a dose of 3 g/kg. Blood was collected from the retro‑orbital plexuses 30, 60, 90, 120 and 180 min after the glucose administration. Serum was separated and used for the estimation of glucose by glucose kit [17].
Induction of diabetes
MCC (400 mg/kg) showed good antihyperglycaemic activity in oral glucose tolerance test (OGTT) and hence was selected for this study. Experimental diabetes was induced in overnight fasted rats by a single intraperitoneal injection of freshly prepared STZ (60 mg/kg body weight) in 0.1 M chilled citrate buffer (pH 4.5) (day 0). Fourteen days after STZ administration, rats with non‑fasting serum glucose more than 300 mg/dl were selected for study. Then they were divided into six groups of six rats each.
Group I: Normal control (nondiabetic), Group II: Diabetic control (1st‑14th day after STZ injection) (2 weeks), Group III: Diabetic control (1st‑21st days after STZ injection) (3 weeks), Group IV: Diabetic control (1st‑28th days after STZ injection) (4 weeks), Group V: Dosing of MCC (400 mg/kg) from 14th to 21st day after STZ injection (1 week treatment), Group VI: Dosing of MCC (400 mg/kg) from 14th to 28th day after STZ injection (2 week treatment).
At the end of the respective period, blood was collected from retro‑orbital plexus, before and after 16 h fasting; care was taken to avoid coprography during 16 h fasting. Then the animals were sacrificed, stomachs were removed and assessed for ulcer score. Mucosa was then scraped and following biochemical parameters were assessed: Total proteins were estimated by Folin–Ciocalteu’s reagent using Bovine serum albumin type IV as standard [18], total hexose were estimated by orcinol reagent using D‑galactose as the standard [19], total fucose were estimated by cysteine HCl using l‑fucose as standard [19], total hexosamines were estimated by indole reagent using D‑glucosamine as standard [20], sialic acid was estimated using thiobarbituric acid using n‑acetyl neuraminic acid as standard [21], cellular ATPase enzymes (Na+-K+ ATPase, Mg2+ ATPase, and Ca2+ ATPase) [22], tumour necrosis factor‑α (TNF‑α), Thiobarbituric acid reactive substances (TBARS), reduced glutathione (GSH) and catalase (CAT) levels [4]. A different set of animals were used as per the above procedure to study formation of the AGE compounds and histopathology in whole stomach [23] and mRNA expression of epidermal growth factor in gastric mucosae [24].
mRNA expression of epidermal growth factor in gastric mucosa
The stomachs were removed and mucosal scrapings were done using a glass slide and immediately stored at −80° until analysis. Total RNA was extracted from mucosal samples using TRIzol® (Invitrogen, Life Technologies Inc., Carlsbad, CA, USA). Following precipitation, RNA was resuspended in RNase free water and its concentration was estimated by absorbance at a 260 nm wavelength. RNA samples were stored at −80° until analysis. Single stranded cDNA was generated from 4 μg of total cellular RNA using ReverAid™ H Minus First Strand cDNA synthesis Kit (Fermentas Life Sciences, USA).
The polymerase chain reaction mixture was amplified in a DNA thermal cycler (Mastercycler, Eppendorf, Germany) at the specifications described in Table 1. The nucleotide sequence of the primers for epidermal growth factor (EGF) and glyceraldehyde 3‑phosphate dehydrogenase (Table 1) was based on the sequences of the published cDNAs [24]. These primers were synthesised by Operon technologies. Polymerase chain reaction products were detected by electrophoresis on a 1% agarose gel containing ethidium bromide. Location of the predicted products was confirmed by using a 25‑bp ladder (Fermentas Life Sciences) as a standard size marker.
| Primer | Sequence | Annealing temperature | bp |
|---|---|---|---|
| EGF | 5’-GTCGTACGATGGGTACTGCCTC-3’ | 57 | 136 |
| 5’-GCGCAGCTTCCACCAACGTAAG-3’ | |||
| GAPDH | 5’-CAAGGTCATCCATGACAACTTTG-3’ | 57° | 480 |
| 5’-GTCCACCACCCTGTTGCTGTAG-3’ |
EGF=Epidermal growth factor, GAPDH=Glyceraldehyde 3‑phosphate dehydrogenase, RT-PCR=Reverse transcription polymerase chain reaction
Table 1: The Nucleotide Sequence Of Primers For Rt Pcr Employed In The Study
Statistical analysis
All the results are expressed as mean±SD. For multiple comparisons, one‑way analysis of variance (ANOVA) was used. When ANOVA showed significant difference (P<0.05), posthoc analysis was performed with Tukey’s multiple comparison test (P<0.05). Statistics was applied using Graphpad Prism 4 software (Graphpad Software Inc., USA).
Results
Single oral dose of MCC 2000 mg/kg body weight did not cause any morbidity or mortality in rats during the 14 days of observation period and was found to be safe. Qualitative analysis of MCC showed the presence of essential oils, phenolics, steroids and flavonoids. The total phenolic and flavonoid content in MCC was expressed in quercetin equivalent (QE) and was found to be 11.73 and 4.4 QE μg/ml, respectively. HPTLC was performed to determine flavonoid glycosides. A bunch of two peaks related to luteoline and some closely related glycosides were identified. The combined area of two peaks was used for the estimation of total flavonoids and was found to be 51.87% w/w.
In vitro antioxidant activity of MCC was determined by DPPH and superoxide radical scavenging assays. It was observed that MCC scavenges DPPH and superoxide radical in a dose dependant manner. 50% inhibitory concentration (IC50) value of MCC for inhibition of DPPH and superoxide was found to be 300 and 1131 μg/ml, respectively.
MCC (200‑1400 μg/ml) inhibited the glycation of BSA and subsequent formation of fluorescent glycation products in a concentration dependent manner. IC50 value of MCC for inhibition of AGE and pentosidine compounds was found to be 1170 and 320 μg/ml, respectively.
Administration of MCC at different dose (100, 200 and 400 mg/kg) levels prevented increase in the glucose levels significantly at all time points (Table 2). MCC 400 mg/kg gave best results in OGTT and hence was selected for further studies in diabetic rats.
| Time (min) | % Increase in serum glucose | ||||
|---|---|---|---|---|---|
| Vehicle control | MCC 400 mg/kg | MCC 200 mg/kg | MCC 100 mg/kg | ||
| 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 30 | 76.07 ± 16.53 | 25.08 ± 16.58* | 27.50 ± 13.81* | 41.75 ± 31.36 | |
| 60 | 103.73 ± 26.37 | 32.41 ± 15.60* | 33.04 ± 6.44* | 54.93 ± 22.83* | |
| 90 | 93.77 ± 30.23 | 31.78 ± 20.27* | 31.29 ± 17.82* | 52.63 ± 20.73* | |
| 120 | 79.10 ± 23.54 | 10.80 ± 4.99* | 34.51 ± 11.53* | 43.54 ± 13.72* | |
| 180 | 52.95 ± 24.05 | 14.59 ± 10.31* | 30.94 ± 13.38* | 32.56 ± 21.01* | |
MCC=Methanol extract of Cuminum cyminum, The data are expressed in mean±SD: n=6,*P<0.05 as compared to vehicle control group
Table 2: Effect Of Methanol Extract Of Cuminum Cyminum On Serum Glucose Levels In Oral Glucose Tolerance Test
In normal rats 16 h fasting caused no macroscopic damage or hemorrhagic lesions (Table 3) whereas, 16 h fasting in diabetic rats, caused severe hemorrhagic gastric lesion in the glandular part of the stomach. The change in blood glucose levels (ΔBGL=nonfasting–BGL fasting) in all the diabetic groups was found to be significantly high as compared to normal control (nondiabetic) rats. Treatment with MCC (400 mg/kg) significantly reduced ΔBGL and ulcer score. Treatment of MCC for 14 days showed significantly more protection than 7 days treatment.
| Groups | Treatment schedule | Glucose levels (mg/dl) | Ulcer score | ||
|---|---|---|---|---|---|
| Before fasting BGL | After fasting BGL | Difference in BGL | |||
| Normal control | Nondiabetic control | 91.12 ± 5.83 | 88.44 ± 6.57 | 2.68 ± 3.12 | 0.0 ± 0.0 |
| 2 weeks diabetic | 1st-14st day after STZ administration, diabetic control | 469.19 ± 35.10* | 258.14 ± 17.06* | 211.04 ± 49.02* | 2 ± 0.44* |
| 3 weeks diabetic | 1st-21st day after STZ administration, diabetic control | 438.89 ± 45.83* | 231.08 ± 26.95* | 207.81 ± 37.81* | 3.28 ± 0.56* |
| 4 weeks diabetic | 1st-28th day after STZ administration, diabetic control | 419.05 ± 49.38* | 217.36 ± 16.88* | 201.69 ± 46.65* | 3.14 ± 0.37* |
| 1 week MCC t/t | 14st-21st day after STZ administration | 395.38 ± 38.93 | 219.55 ± 10.44 | 175.82 ± 34.15 | 2.33 ± 0.51# |
| 2 week MCC t/t | 14st-28th day after STZ administration | 306.88 ± 43.11@ | 159.36 ± 19.09@ | 147.52 ± 31.99@$ | 1.5 ± 0.5@$ |
Each value is the mean±SD of six determinations,*P<0.05 as compared to normal control, #P<0.05 as compared to 3 week diabetic control group, @P<0.05 as compared to 4 week diabetic control group, $P<0.05 as compared to 1 week (14th day of STZ administration to 21st day of STZ administration) MCC treatment. BGL=Blood glucose levels, STZ=Streptozotocin
Table 3: Effect Of Duration Of Diabetes And Methanol Extract Of Cuminum Cyminum Treatment On Blood Glucose Levels And Ulcer Score
In normal control rats 16 h fasting did not caused loss of gastric mucosal glycoproteins. Total carbohydrates:total proteins (TC:TP) ratio in normal (nondiabetic) rats was found to be 1.01±0.10 (Table 4). Whereas, in diabetic rats, 16 h fasting caused heavy loss of gastric mucosal glycoproteins as compared to normal control (nondiabetic) rats. The loss in glycoproteins was also found to be dependent on the duration of diabetes (Table 4). The loss in glycoproteins was mainly due to decreases in the concentration of individual carbohydrates. Treatment with MCC significantly prevented loss in mucosal glycoproteins and restored TC:TP ratio (Table 4) as compared to respective diabetic control rats. Treatment with MCC significantly increased hexosamines and sialic acid concentration, which are mainly responsible for the viscous nature of gastric mucus.
| Groups | Treatment schedule | Mucosal parameter (µg/100 mg of tissue) | ||||||
|---|---|---|---|---|---|---|---|---|
| Proteins | Total hexosamine | Sialic acid | Total hexoses | Total fucose | TC | TC:TP | ||
| Normal Control | Nondiabetic control | 9281.8 ± 471.35 | 3847.8 ± 588.65 | 174.2 ± 26.37 | 5093.0 ± 185.90 | 307.8 ± 49.92 | 9423.8 ± 543.75 | 1.01 ± 0.10 |
| 2 weeks diabetic | 1st-14th day after STZ administration, diabetic control | 9973.95 ± 806.10 | 2249.76 ± 453.36 | 144.08 ± 18.48 | 3934.72 ± 561.88* | 159.35 ± 13.99* | 6487.92 ± 879.31* | 0.65 ± 0.09* |
| 3 weeks diabetic | 1st-21st day after STZ administration, diabetic control | 10648.24 ± 1532.39* | 1492.34 ± 340.78* | 91.08 ± 26.19* | 1908.09 ± 202.82* | 149.19 ± 48.65 | 3640.72 ± 496.05* | 0.34 ± 0.06* |
| 4 weeks diabetic | 1st-28th day after STZ Administration, Diabetic control | 10998.66 ± 1177.10* | 1246.49 ± 73.06* | 94.69 ± 10.98* | 1675.81 ± 203.74* | 102.74 ± 20.75* | 3119.74 ± 255.25* | 0.28 ± 0.03* |
| 1 week MCC t/t | 14th-21st day after STZ administration | 8076.10 ± 784.82# | 2629.08 ± 378.41# | 107.71 ± 7.22 | 3202.22 ± 450.63# | 246.35 ± 33.76# | 6185.37 ± 364.43# | 0.76 ± 0.04# |
| 2 week MCC t/t | 14th-28th day after STZ administration | 6541.43 ± 1231.49@ | 2534.20 ± 454.15@ | 111.97 ± 11.16@ | 3823.19 ± 1181.65@ | 222.52 ± 25.74@ | 6691.89 ± 348.02@ | 0.94 ± 0.11@,$ |
Each value is the mean±SD of six determinations,*P<0.05 as compared to normal control, #P<0.05 as compared to 3 week diabetic control group, @P<0.05 as compared to 4 week diabetic control group, $P<0.05 as compared to 1 week (14th day of STZ administration to 21st day of STZ administration) MCC treatment, TC=Total carbohydrates, TP=Total proteins, STZ=Streptozotocin, MCC=Methanol extract of cuminum cyminum
Table 4: Effect Of Duration Of Diabetes And Mcc Treatment On Gastric Mucosal Glycoprotein
Diabetes caused a significant increase in the mucosal lipid peroxidation product (malondialdehyde (MDA)) levels as compared to normal control (nondiabetic) rats. Diabetes also caused a significant decrease in the mucosal antioxidant enzyme levels (GSH and CAT) (Table 5). MCC treatment significantly reduced MDA levels and increased GSH, CAT enzyme levels when compared to respective diabetic controls (Table 5).
| Groups | Treatment schedule | Gastric mucosal | ||
|---|---|---|---|---|
| GSH (µ moles of GSH/mg protein) | Catalase (units/ml) | TBARS (n moles of MDA/mg Protein) | ||
| Normal control | Nondiabetic control | 24.32 ± 1.97 | 384.52 ± 24.91 | 0.65 ± 0.018 |
| 2 weeks diabetic | 1st-14th day after STZ administration, diabetic control | 23.83 ± 1.55 | 350.11 ± 66.86 | 0.68 ± 0.20 |
| 3 weeks diabetic | 1st-21st day after STZ administration, diabetic control | 18.25 ± 2.91* | 299.53 ± 58.30* | 0.73 ± 0.05* |
| 4 weeks diabetic | 1st-28th day after STZ administration, diabetic control | 15.88 ± 1.69* | 277.13 ± 49.31* | 1.82 ± 0.41* |
| 1 week MCC t/t | 14th-21st day after STZ administration | 16.73 ± 1.43 | 363.93 ± 58.30# | 0.70 ± 0.10 |
| 2 week MCC t/t | 14th-28th day after STZ administration | 19.38 ± 1.80@ | 375.86 ± 77.34@ | 1.41 ± 0.16@ |
Each value is the mean±SD of six determinations,*P<0.05 as compared to normal control, #P<0.05 as compared to 3 week diabetic control group, @P<0.05 as compared to 4 week diabetic control group. MCC treatment, STZ=Streptozotocin, GSH=Glutathione, TBARS=Thiobarbituric acid reactive substances, MDA=Malondialdehyde
Table 5: Effect Of Duration Of Diabetes And Methanol Extract Of Cuminum Cyminum Treatment On Gastric Mucosal Glutathione, Catalase And Tbars Levels
Cellular ATPase enzymes are the indicator for membrane integrity. Diabetes caused a significant decrease in the cellular ATPase enzymes levels as compared to normal control rats (Table 6). MCC treatment for 1 and 2 week to diabetic rats prevented the loss of ATPase activity significantly as compared to their respective controls.
| Groups | Treatment schedule | Cellular ATPase (μ moles of Pi liberated/mg protein) | ||
|---|---|---|---|---|
| Na+-K+ ATPase | Mg+ ATPase | Ca+ ATPase | ||
| Normal control | Nondiabetic control | 85.70 ± 4.39 | 64.75 ± 16.76 | 61.58 ± 2.94 |
| 2 weeks diabetic | 1st-14th day after STZ administration, diabetic control | 79.52 ± 10.96 | 51.74 ± 3.45 | 55.59 ± 9.01 |
| 3 weeks diabetic | 1st-21st day after STZ administration, diabetic control | 64.77 ± 2.82* | 39.62 ± 9.09* | 52.10 ± 5.38* |
| 4 weeks diabetic | 1st-28th day after STZ administration, diabetic control | 53.65 ± 7.35* | 35.97 ± 7.39* | 47.58 ± 5.77* |
| 1 week MCC t/t | 14th-21st day after STZ administration | 88.74 ± 9.16# | 66.27 ± 15.27# | 75.91 ± 5.11# |
| 2 week MCC t/t | 14th-28th day after STZ administration | 92.26 ± 7.53@ | 71.63 ± 17.34@ | 76.00 ± 6.18@ |
Each value is the mean±SD of six determinations,*P<0.05 as compared to normal control, #P<0.05 as compared to 3 week diabetic control group, @P<0.05 as compared to 4 week diabetic control group. MCC=Methanol extract of Cuminum cyminum, STZ=Streptozotocin
Table 6: Effect Of Duration Of Diabetes And Methanol Extract Of Cuminum Cyminum Treatmenton Gastric Mucosal Cellular Atpase Enzyme Levels
TNF‑α was chosen as a marker for PARP pathway. 1 and 2 week treatment with MCC to diabetic rats significantly inhibited TNF‑α levels as compared to their respective diabetic controls (Table 7).
| Groups | TNF-α (pg/ml) |
|---|---|
| 2 weeks diabetic | 7.744 ± 1.212 |
| 3 weeks diabetic | 8.270 ± 1.720 |
| 4 weeks diabetic | 7.932 ± 1.824 |
| 1 week MCC treatment | 6.084 ± 0.588* |
| 2 week MCC treatment | 4.139 ± 1.635# |
Each value is the mean±SD of four determinations,*P<0.05 as compared to 3 week diabetic control group, #P<0.05 as compared to 4 week diabetic control group. MCC=Methanol extract of cuminum cyminum, TNF‑α=Tumor necrosis factor‑α.
Table 7: Effect Of Duration Of Diabetes And Methanol Extract Of Cuminum Cyminum Treatment On Gastric Mucosal Tnf-α Levels
In this study, AGEs were measured using the intensity of fluorescence, which is one of their characteristics and expressed as arbitrary (fluorescence) units/mg protein content. AGE related fluorescence in the stomach was found to be significantly increased and highest during the 4 weeks of diabetes as compared to normal control rats. Administration of MCC (400 mg/kg) for 2 weeks (14th‑28th day after STZ injection) significantly prevented formation of AGE compounds as compared to 4 week diabetic control rats (Table 8).
| Groups | Age (arbitary unit/mg protein) |
|---|---|
| Normal control | 3.01 ± 0.18 |
| 4 weeks diabetic | 8.40 ± 0.40* |
| 2 weeks MCC treatment | 5.41 ± 0.48*,# |
Each value is the mean±ΣD of six determinations,*P<0.05 as compared to normal control group, #P<0.05 as compared to 4 week diabetic control group. MCC=Methanol extract of Cuminum cyminum.
Table 8: Effect Of Diabetes And Methanol Extract Of Cuminum Cyminum Treatment Onstomach Age Levels
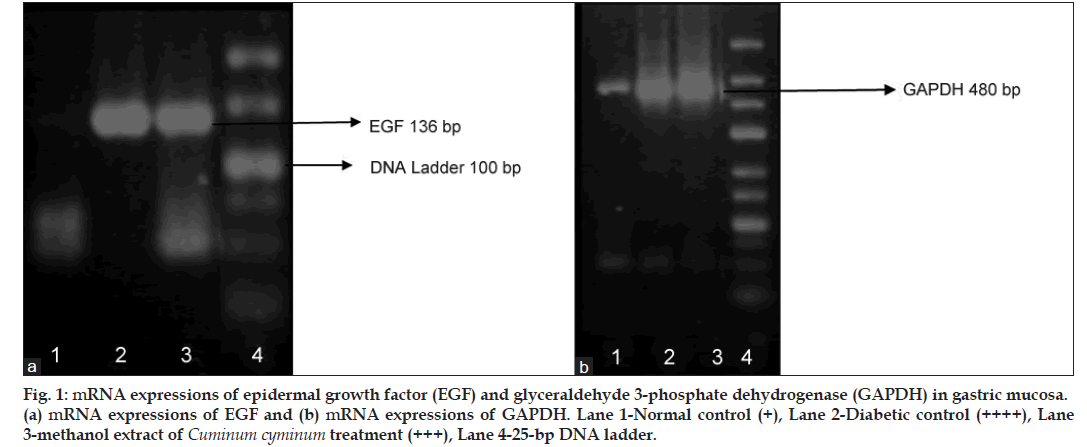
Reverse transcription polymerase chain reaction (RT-PCR) studies for EGF gene expression revealed the absence of its expression in the stomach of normal rats. Whereas in 4 week diabetic control, a well‑defined signal (a thick band) was seen indicating EGF expression. In MCC treated rats the expression was less compared to diabetic control stomachs, as indicated by the difference in the thickness and intensity of bands (fig. 1).
Fig 1: mRNA expressions of epidermal growth factor (EGF) and glyceraldehyde 3‑phosphate dehydrogenase (GAPDH) in gastric mucosa. (a) mRNA expressions of EGF and (b) mRNA expressions of GAPDH. Lane 1‑Normal control (+), Lane 2‑Diabetic control (++++), Lane 3‑methanol extract of Cuminum cyminum treatment (+++), Lane 4‑25‑bp DNA ladder.
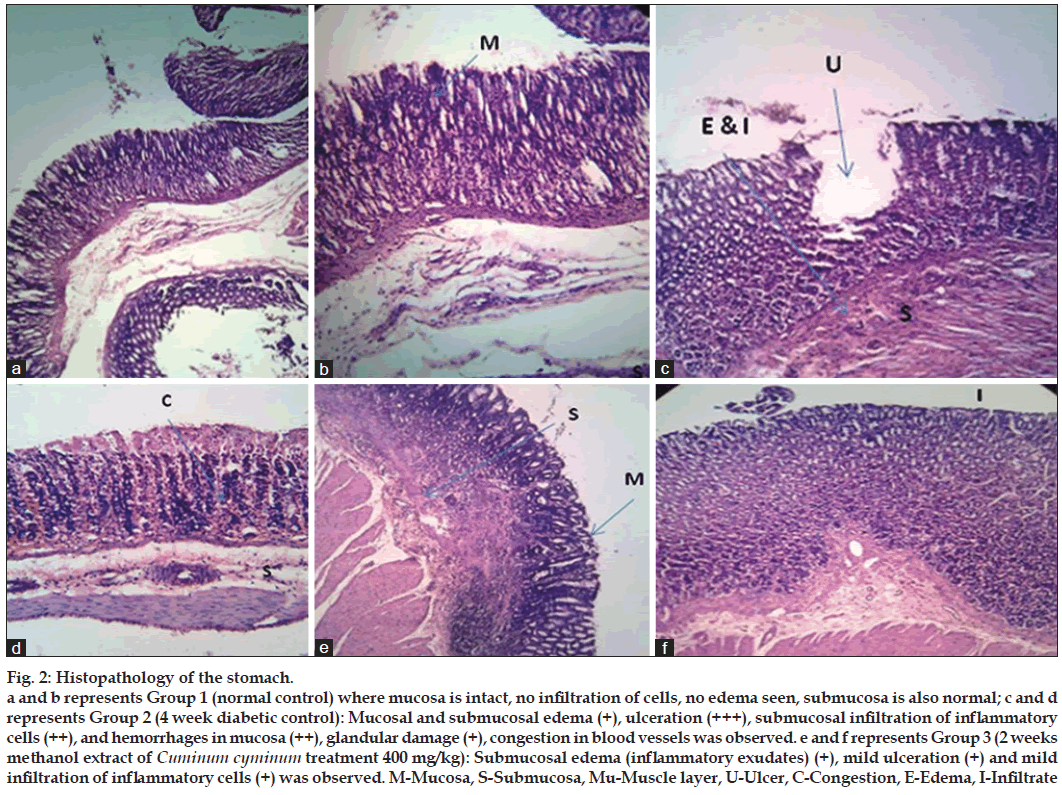
Microscopic examination (fig. 2) of the stomach from the normal control rats did not reveal any lesion of pathological significance. Stomach of 4 week diabetic control showed mucosal and mild submucosal edema, severe ulceration, moderate submucosal infiltration of inflammatory cells, mucosal congestion and hemorrhages in mucosa with mild glandular damage. However, 2 weeks MCC treatment revealed considerable recovery with mild, submucosal edema (inflammatory exudates) ulceration and mild infiltration of inflammatory cells was observed.
Discussion
This study is a first attempt in understanding the pathogenesis of gastric ulceration in diabetes by alternate biochemical pathways using suitable markers for each pathway. The four major biochemical pathways studied, leading to diabetes related complications are polyol pathway; the hexosamine pathway; excess/inappropriate activation of PKC isoforms and accumulation of advanced glycation end products. Each of these pathways is reported to be injurious, but collectively, they cause an imbalance and may lead to excess formation of ROS. Increased ROS stress within the cell leads to activation of the PARP pathway, which is known to regulate the events leading to inflammatory reactions.
In diabetic control rats, fasting for 16 h itself caused severe gastric lesions, severe loss of gastric mucosal glycoproteins and significant change in blood glucose levels (ΔBGL=nonfasting BGL–fasting BGL) as compared to normal control (nondiabetic) rats. These results are in concurrence with the earlier findings of Igarashi et al. [6]. Fasting caused a significant change in the BGL with a profound hypoglycaemic response reducing GMBF, which is essential for delivery of glucose (precursor for glycoprotein synthesis). Hence, during fasting, there must have been decrease in the GMBF leading to depletion of gastric mucus and hence gastric ulceration was observed. Though there was no significant difference in ΔBGL among the diabetic groups (2, 3 and 4 weeks diabetic) there was an increase in the ulcer score with the duration of diabetes, indicating that the gastric ulcer formation not only depends on profound hypoglycaemic responses but also depends on duration of diabetes.
Treatment with MCC significantly reduced blood glucose levels and significantly controlled ΔBGL; this indicates strict glycemic control, an essential parameter to reduce the risk of diabetic complications. MCC treatment also prevented fasting induced gastric ulcer formation and prevented the loss in glycoprotein content of gastric mucus as evidenced by higher TC:TP ratio. Hence, MCC treatment might have increased GMBF which is essential for gastric mucus production.
Formation of AGE related compounds in the stomach increased with the duration of diabetes and were significantly higher than the normal control rats (nondiabetic). Progressive deposition of glycated products leads to thickening of blood vessels and mucosal capillary closures related to microthrombosis. Capillary closures lead to decrease in GMBF, which is essential for delivery of glucose (precursor for glycoprotein synthesis), oxygen, nutrients, removal of toxic substances and maintenance of the relatively high pH microenvironment. Previous studies have also reported the decrease in GMBF in diabetic state as one of the reasons for gastric ulcer. This indicates that formation of AGE compounds leads to capillary closures and decreased GMBF leading to decreased gastric mucus production.
Treatment of MCC for 2 weeks significantly inhibited AGE formation as compared to the 4 week diabetic control rats. MCC also inhibited the in vitro formation of AGE and pentosidine compounds in a dose dependent manner. Thus MCC might have inhibited AGE formation in gastric mucosal capillaries and increased GMBF thereby reducing the chances of ulcer production.
ATPase enzymes are the marker for the cellular integrity and hence loss in ATPase activity indicates loss in cellular integrity. ATPase enzymes were chosen as the markers for PKC pathway. In diabetes, the PKC pathway is activated. PKCs regulate the biosynthesis of proinflammatory cytokines, nitric oxide (NO), and ROS as well as activate phospholipase A2 (PLPA2). They specially hydrolyse the acyl chain at the second position of the glycerol moiety of phospholipids and disrupt the phospholipid layer of gastric mucus. It also inactivates membrane bound ATPase enzymes viz Na+-K+ ATPase, Ca+ ATPase and Mg+ ATPase [25]. Diabetes caused significant reduction in the cellular ATPase enzymes levels as compared to normal control rats (nondiabetic), which indicates that diabetes makes the gastric mucosal surface more fragile and susceptible to stress. Treatment with MCC significantly increased cellular ATPase activity as compared to respective diabetic control. Hence one of the mechanism by which MCC ameliorates gastric ulcer formation in diabetes is preventing PKC pathway induced loss in cellular ATPase activities.
ROS like superoxide radical anion and hydroxyl radical are now considered one of the major causative factors for mucosal lesions through oxidative stress. The radicals promote mucosal damage by causing degradation of the epithelial basement membrane components, complete alteration of the cell metabolism and DNA damage [26]. GSH and CAT (antioxidant enzymes) scavenges the free radicals, thereby preventing ROS mediated gastric damage. They also maintain mucosal integrity and were selected as the marker for ROS mediated gastric damage. Diabetes caused a significant decrease in the GSH and CAT levels. Thus decrease in the antioxidant enzyme levels is also one of the mechanisms of gastric ulcer formation in diabetes. Treatment with MCC significantly increased GSH and CAT concentration with respect to 4 week diabetic control rats. The mechanism is attributed to increase in the endogenous defensive antioxidant status.
TNF‑α is a proinflammatory cytokine, which induces severe inflammatory reaction leading to gastric damage. Along with nuclear transcription factor (NF‑κB) it induces AGE as well PKC pathway. NF‑κB is responsible for ulcer recurrence. TNF‑α and TBARS levels were chosen as marker for the PARP pathway. Progressively, TNF‑α levels and lipid peroxidation products (MDA) levels increased with the duration of diabetes histopathology of stomach of diabetic rats showed the presence of heavy infiltrates of neutrophils. Neutrophilis have been reported to stimulates TNF‑α activity. PARP pathway along with ROS is also involved in the pathogenesis of gastric ulcer in diabetic state. MCC treatment significantly reduced TNF‑α level and thus prevented TNF‑α mediated gastric mucosal damage and reduced MDA levels. Thus, MCC prevents gastric ulcer formation by ameliorating PARP pathway.
EGF is one of the important peptide growth factors that accelerates epithelial cell migration necessary for reepithelialization of the ulcer base, and also triggers cell proliferation and divisions crucial for filling the mucosal defect (ulcer crater), thus enabling reconstruction of epithelial structures within the ulcer scar. EGF exerts these actions by binding to its receptor epidermal growth factor receptor (EGFR) on the epithelial cell surface. Reports in the literature suggest that EGFR is elevated in the gastric mucosa [27] and EGFR‑signalling pathways appear to be sensitizing the diabetic gastric mucosa to damaging agents [27]. The increase in EGFR signalling has been reported to be activated by ROS.
This study demonstrated that the gastric mucosa of normal rats (nondiabetic) failed to express mRNA of EGF. This result is in concurrence of earlier reported results. Whereas increased mRNA expression of EGF was observed in the gastric mucosa of diabetic control rats (4 week diabetic control rats). The up‑regulated expression of EGF is likely to be involved in the recovery of gastric mucosa from continuous damage due to hyperglycaemia induced ROS activation. 2 week MCC treatment (14th‑28th days after STZ injection) showed decreased mRNA expression of EGF. These effects might be due to ROS scavenging action and decreased TNF‑α level, where both are reported to activate EGF and its EGFR signalling pathways.
To summarise, Ayurvedic treatment forms an important part of adjunct therapy to conventional antidiabetic treatment. Seeds of C. cyminum have been reported to contain many important phytoconstituents like essential oils, phenolic compounds, steroids and flavonoids. This study shows that MCC has been able to reduce hyperglycemia, oxidative stress, and formation of AGE. Thus, MCC may serve as an adjunct to conventional antidiabetic treatment, which may help in attenuating diabetic complications.
Our study concludes that the pathogenesis of gastric ulcers occurring in the diabetic state is multifactorial and involves various alternate biochemical pathways. Each of these pathways is acting alone or synergistically, producing continuous gastric damage making it more fragile towards stress and noxious agents. Treatment with MCC prevented diabetic gastro complications by ameliorating the various biochemical pathways, mainly by increasing GMBF, antioxidant enzymes, cellular ATPase enzyme levels.
Acknowledgements
The authors thank Dr. Aashish S. Phadke, M.D. (Ayur), for authenticating seeds of C. cyminum.
References
- Thévenod F. Pathophysiology of diabetes mellitus type 2: Roles of obesity, insulin resistance and β-cell dysfunction. In: Masur K, Thévenod F, Zänker KS, ediors. Diabetes and Cancer. Epidemiological Evidence and Molecular Links, Front Diabetes. Vol. 19. Basel: Karger Publications; 2008. p. 1-18.
- Cumbie BC, Hermayer KL. Current concepts in targeted therapies for the pathophysiology of diabetic microvascular complications. Vasc Health Risk Manag 2007;3:823-32.
- Bytzer P, Talley NJ, Leemon M, Young LJ, Jones MP, Horowitz M. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: A population-based survey of 15,000 adults. Arch Intern Med 2001;161:1989-96.
- Joshi MC, Dorababu M, Prabha T, Kumar MM, Goel RK. Effects of Pterocarpus marsupium on NIDDM-induced rat gastric ulceration and mucosal offensive and defensive factors. Indian J Pharmacol 2004;36:296-302.
- Takeuchi K, Ueshima K, Ohuchi T, Okabe S. Induction of gastric lesions and hypoglycemic response by food deprivation in streptozotocin-diabetic rats. Dig Dis Sci 1994;39:626-34.
- Igarashi S, Kume E, Narita H, Kinoshita M. Food deprivation depletes gastric mucus glycoprotein in streptozotocin-induced diabetic rats. Jpn J Pharmacol 2000;84:51-5.
- Dhandapani S, Subramanian VR, Rajagopal S, Namasivayam N. Hypolipidemic effect of Cuminum cyminum L. on alloxan induced diabetic rats. Pharmacol Res 2002;46:251-5.
- Roman-Ramos R, Flores-Saenz JL, Alarcon-Aguilar FJ. Anti-hyperglycemic effect of some edible plants. J Ethnopharmacol 1995;48:25-32.
- Lee HS. Cuminaldehyde: Aldose reductase and alpha-glucosidase inhibitor derived from Cuminum cyminum L. seeds. J Agric Food Chem 2005;53:2446-50.
- Khandelwal KR. Phytochemical evaluation. In, Practical Pharmacognosy Techniques and Experiments. 12th ed. Pune; Nirali Prakashan: 2004.
- Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substances and antioxidants by means of Folin–Chiocalteu reagent. Methods Enzymol 1999;99:152-78.
- Zhishen J, Mengeheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 1999;64:555-6.
- Shirke SS, Jadhav SR, Jagtap AG. Methanolic extract of Cuminum cyminum inhibits ovariectomy-induced bone loss in rats. Exp BiolMed (Maywood) 2008;233:1403-10.
- Nishimiki M, Rao NA, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulphate and molecular oxygen. Biochem Biophys Res Commun 1972;46:849-53.
- Blois MS. Antioxidant determination by the use of free radical. Nature 1958;4:1200-1.
- Sakai M, Oimomi M, Kasuga M. Experimental studies on the role of fructose in the development of diabetic complications. Kobe J Med Sci 2002;48:125-36.
- Prakasam A, Sethupathy S, Pugalendi KV. Effect of Casearia esculenta root extract on blood glucose and plasma antioxidant status in streptozotocin diabetic rats. Pol J Pharmacol 2003;55:43-9.
- Lowry OH, Rosebrough NJ, Farr Al, Randall RJ. Protein measurementwith the Folin phenol reagent. J Biol Chem 1951;193:265-75.
- Anoop A, Jegadeesan M. Biochemical studies on the antiulcerogenic potential of Hemidesmus indicus R.Br. var. indicus. J Ethnopharmacol 2003;84:149-56.
- Dische Z, Borenfreund E. A spectrophotometric method for the microdetermination of hexosamines. J Biol Chem 1950;184:517-22.
- Warren L. The thiobarbituric acid assay of sialic acids. J Biol Chem 1959;234:1971-5.
- Iheanyichukwu E, Michael OM, Emmanuel OA. In vitro effects of aqueous extracts of Zanthoxylum macrophylla roots on adenosine triphosphatases from human erythrocytes of different genotypes. Biokemistri 2005;17:19-25.
- Nakagawa T, Yokozawa T, Terasawa K, Nakanishi K. Therapeutic usefulness of Keishi-bukuryo-gan for diabetic nephropathy. J Pharm Pharmacol 2003;55:219-27.
- Ye YN, So HL, Liu ES, Shin VY, Cho CH. Effect of polysaccharides from Angelica sinensis on gastric ulcer healing. Life Sci 2003;72:925-32.
- Berstad AE, Berstad K, Berstad A. pH-activated phospholipase A2: An important mucosal barrier breaker in peptic ulcer disease. Scand J Gastroenterol 2002;37:738-42.
- Schraufstätter I, Hyslop PA, Jackson JH, Cochrane CG. Oxidant-induced DNA damage of target cells. J Clin Invest 1988;82:1040-50.
- Khan AJ, Fligiel SE, Liu L, Jaszewski R, Chandok A, Majumdar AP. Induction of EGFR tyrosine kinase in the gastric mucosa of diabetic rats. Proc Soc Exp Biol Med 1999;221:105-10.