- *Corresponding Author:
- Qi Guo
Department of Radiotherapy, Second Affiliated Hospital of Soochow University, Suzhou, Jiangsu Province 215006, China
E-mail: guoqi456258@163.com
| Date of Received | 24 Decenmber 2021 |
| Date of Revision | 18 October 2022 |
| Date of Acceptance | 05 July 2023 |
| Indian J Pharm Sci 2023;85(4):972-978 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Previous reports have indicated that walnut green husk extract has an inhibitory effect on human cancer development. This project aimed to explore the influence of walnut green husk extract on ovarian cancer. SKOV3 cells were exposed to various doses of walnut green husk extract or transfected with microRNAcontrol or microRNA-144-3p. Meanwhile, anti-microRNA-144-3p or anti-microRNA-control-transfected SKOV3 cells were treated with high-dose walnut green husk extract. Cell counting kit-8, flow cytometry and Transwell monitored cell activity, cycle progression, migration and invasion. Cyclin D1, matrix metallopeptidase 2, matrix metallopeptidase 9, microRNA-144-3p contents were appraised via Western blot or real-time quantitative polymerase chine reaction. After treatment with different concentrations of walnut green husk extract, cell activity, cycle progression, migration, invasion and cyclin D1, matrix metallopeptidase 2 and matrix metallopeptidase 9 protein expression were decreased and microRNA-144-3p level increased in a dose-dependent manner. MicroRNA-144-3p upregulation might hinder cell activity, cycle progression, migration and invasion. Additionally, miR-144-3p knockdown might abolish the repression of high-dose walnut green husk extract on Janus kinase 2 and signal transducer, and activator of transcription 3 expression. Walnut green husk extract might block ovarian cancer development via modulating the microRNA-144-3p/ Janus kinase 2/signal transducer and activator of transcription 3 pathway.
Keywords
Walnut green husk extract, ovarian cancer, microRNA-144-3p, proliferation, migration
As a prevalent gynecological malignancy in females, ovarian cancer continues to be mainly treated with surgery and chemotherapy[1]. During recent years, many medicines within Traditional Chinese Medicine (TCM) have been presented to function as effective anti-cancer drugs or adjuvants to alleviate the progression of human tumors[2], including ovarian cancer[3]. Some pharmacological research suggested that Walnut Green Husk (WGH), also known as Qing Long Yi might clear away restrain bacterium, anti-oxidant, anti-tumor and other actions[4,5]. Previous research suggested that Walnut Green Husk Extract (WGHE) might hinder gastric cancer cell proliferation and migration through regulating multiple signal pathways[6]. Moreover, WGHE might kill human prostate carcinoma cells by inducing apoptosis[7]. Apart from that, WGHE might dampen inflammation and apoptosis via the Janus Kinase 2/Signal Transducer and Activator of Transcription 3 (JAK2/STAT3) signaling pathways in rats[8]. However, the impact and mechanism of WGHE on ovarian cancer cells are far from being addressed. Furthermore, a related study verified microRNA (miR)-144-3p was closely related to ovarian cancer progression and implied a new target for clinical treatment[9]. Meanwhile, miR-144-3p impeded ovarian cancer cell growth via regulating Pre-Leukemia Transcription Factor 3 (PBX-3)[10]. Hence, the purpose of this research is to verify whether the influence of WGHE on ovarian cancer development was correlative with miR-144-3p.
Materials and Methods
Reagents:
SKOV3 (American Type Culture Collection (ATCC), Manassas, Virginia, United States of America (USA)) was cultivated in Roswell Park Memorial Institute (RPMI)-1640 medium (Crkbio, Wuhan, China). WGHE was provided by Siyecao (Xian, China). Balb (Beijing, China) supplied Cell Counting Kit-8 (CCK-8) reagent, cell apoptosis detection kit and Radioimmunoprecipitation Assay (RIPA) protein lysates. Primary antibodies like cyclin D1, Matrix Metallopeptidase (MMP)-2, MMP9, JAK2, and STAT3 and secondary antibody (Immunoglobulin G (IgG)-Horseradish Peroxidase (HRP)) were respectively obtained from Fine bio (Wuhan, China) and Amyjet (Wuhan, China). Fswbio (Shanghai, China) provided Transwell chamber and matrix glue. SYBR Premix Ex TaqTM kit was purchased from Khayal (Wuhan, China).
Cell treatments:
SKOV3 cells were routinely cultured in RPMI-1640 medium with WGHE at various doses (10, 30 and 90 μg/ml), recorded as the low dose group (WGHE-L), the medium dose group (WGHE-M), and the high dose group (WGHE-H). Meanwhile, the normal cultured SKOV3 cells were used as the control group. miR-144-3p mimic and miR-con were transfected into SKOV3 cells, which were termed as miR-144-3p or miR-con group. Research conducted transfection of miR-144-3p inhibitor or anti-miR-con into SKOV3 cells, followed by exposure to 90 μg/ml WGHE, namely anti-miR-144-3p+WGHE-H and anti-miR-con+WGHE-H group.
Cell viability assay:
After cells were cultured for 48 h, 10 μl CCK-8 was added for 2 h. At last, samples were monitored under microplate reader.
Flow cytometry:
After being fixed with precooled 70 % ethanol, collected cells in each group were washed and mixed with Ribonucleases (RNase) A for 30 min. Then, cells were reacted with Propidium Iodide (PI) at 4° for 30 min. Finally, the apoptosis cells were analyzed using flow cytometry at 488 nm and Deoxyribonucleic Acid (DNA) cell cycle analysis software.
Transwell assay:
For migration assay, after being starved with serum- free medium for 12 h, 100 μl was inoculated in Transwell upper chamber for 24 h. After discarding, cells were washed, fixed, stained and the upper non-migrating cells were erased. Five fields were observed and counted under microscope. For invasion, Transwell was coated with matrix glue and then subjected to a migration test.
Western blotting analysis:
Total SKOV3 cell proteins were extracted using RIPA buffer and subjected to Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membrane. After blocking, primary antibodies (1:1000) cyclin D1, MMP2, MMP9, JAK2, STAT3, β-actin (internal reference) were respectively added into the membranes, which then were incubated with the secondary antibodies for 2 h. At last, protein bands were detected using Enhanced Chemiluminescence (ECL) and the gray value was analyzed using Image J software.
Real Time-qualitative Polymerase Chain Reaction (RT-qPCR) assay:
Extracted RNA was reverse-transcribed into complementary DNA (cDNA). PCR amplification was implemented using U6 as an internal control. Expression was analyzed via 2-ΔΔCt. miR-144-3p: GCGCGCTACAGTATAGATGATG (sense) and GCTGTCAACGATACGCTACG (antisense). U6: CTCGCTTCGGCAGCACA (sense) and AACGCTTCACGAATTTGCGT (antisense).
Statistical analysis:
Statistical analysis was carried out according to Statistical Package for the Social Sciences (SPSS) 20.0. Difference were considered significant when p<0.05. Measurement data were expressed as mean±standard deviation (x̄±s), and compared with t-test and one-way Analysis of Variance (ANOVA).
Results and Discussion
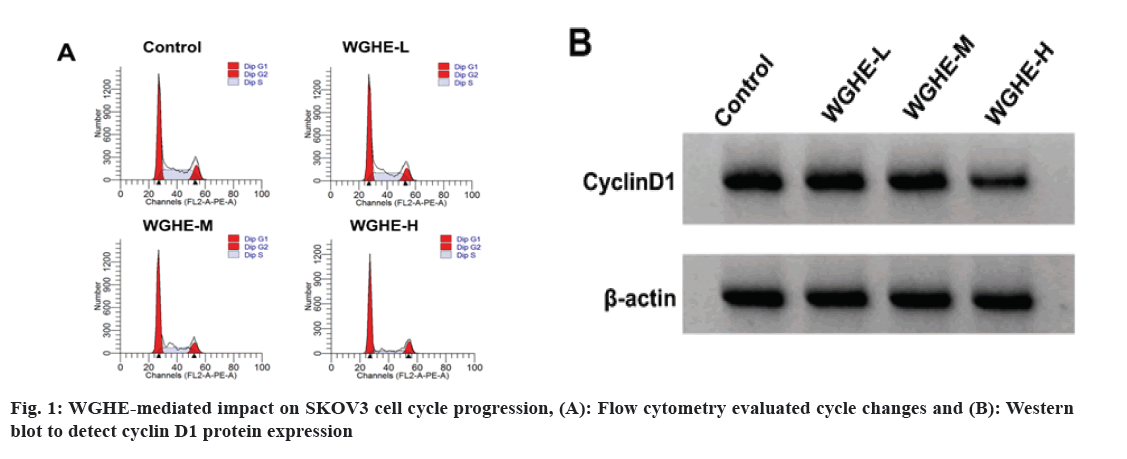
According to the data exhibited in fig. 1 and Table 1, cell viability, cell proportion in S phase, cyclin D1 protein level in WGHE-L, WGHE-M and WGHE-H groups were reduced after WGHE exposure in concentration-dependent ways (p<0.05), whereas the cell proportion was improved in Resting/Growth (G0/G1) phase.
| Group | A value | G0/G1 (%) | S (%) | G2 (%) | Cyclin D1 |
|---|---|---|---|---|---|
| Control | 1.053±0.10 | 45.13±3.86 | 30.85±2.43 | 24.02±2.18 | 0.83±0.08 |
| WGHE-L | 0.903±0.08* | 51.43±4.25* | 24.24±2.05* | 24.33±2.27 | 0.70±0.06* |
| WGHE-M | 0.705±0.07* | 59.06±5.17* | 16.83±1.25* | 24.11±2.03 | 0.57±0.05* |
| WGHE-H | 0.442±0.05* | 62.05±5.43* | 14.07±1.03* | 23.88±1.93 | 0.42±0.03* |
| F | 105.607 | 23.548 | 162.074 | 0.072 | 82.925 |
| p | 0.000 | 0.000 | 0.000 | 0.974 | 0.000 |
Note: *p<0.05, relative to control group
Table 1: The Effect of Different Concentrations of WGHE on SKOV3 Cell Cycle (x̄±s, n=9)
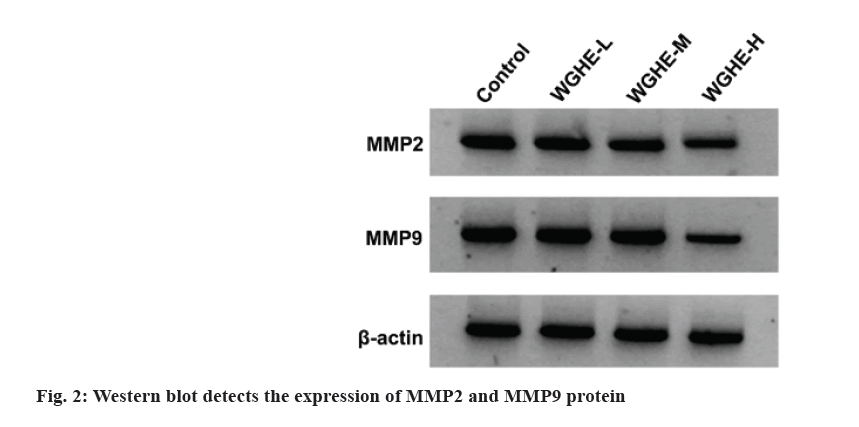
Data displayed migration and invasion number, MMP2 and MMP9 protein expression in WGHE-L, WGHE-M and WGHE-H groups were reduced after WGHE exposure in dose-dependent manners (p<0.05) as shown in fig. 2 and Table 2.
| Group | MMP2 | MMP9 | Number of cell migration | Number of cell invasion |
|---|---|---|---|---|
| Control | 0.83±0.07 | 0.73±0.07 | 236±18.13 | 176±14.02 |
| WGHE-L | 0.68±0.06* | 0.61±0.05* | 183±14.02* | 151±11.17* |
| WGHE-M | 0.51±0.05* | 0.47±0.02* | 147±11.17* | 117±8.25* |
| WGHE-H | 0.38±0.03* | 0.32±0.03* | 103±8.16* | 73±5.17* |
| F | 82.925 | 82.925 | 82.925 | 172.241 |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05, relative to control group
Table 2: Effects of Different Concentrations Of WGHE On SKOV3 Cell Migration and Invasion (x̄±s, n=9)
As presented in Table 3, miR-144-3p level was obviously enhanced in WGHE-L, WGHE-M and WGHE-H groups vs. control group (p<0.05).
| Group | MMP2 | MMP9 | Number of cell migration | Number of cell invasion |
|---|---|---|---|---|
| Control | 0.83±0.07 | 0.73±0.07 | 236±18.13 | 176±14.02 |
| WGHE-L | 0.68±0.06* | 0.61±0.05* | 183±14.02* | 151±11.17* |
| WGHE-M | 0.51±0.05* | 0.47±0.02* | 147±11.17* | 117±8.25* |
| WGHE-H | 0.38±0.03* | 0.32±0.03* | 103±8.16* | 73±5.17* |
| F | 82.925 | 82.925 | 82.925 | 172.241 |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05, relative to control group
Table 3: Wghe Induced Influence on miR-144-3P Content (x̄±s, n=9)
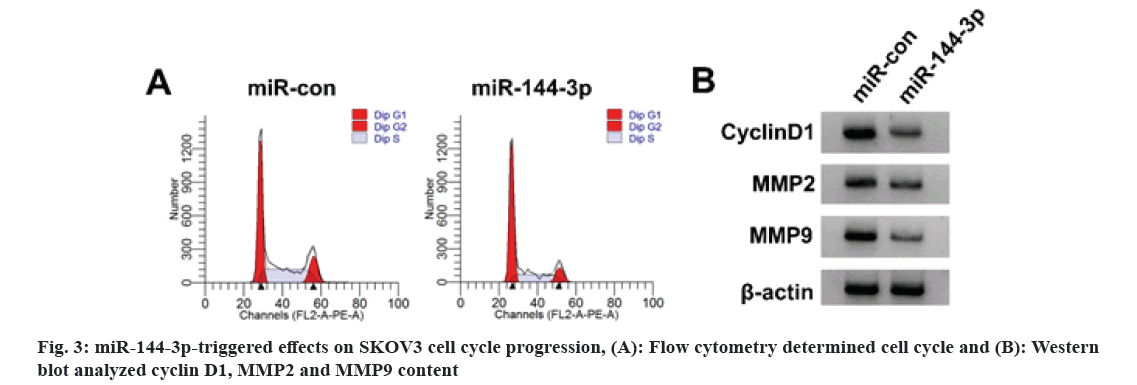
As illustrated in fig. 3 and Table 4, relative to the miR-con group, SKOV3 cell viability, cell proportion in S phase, SKOV3 cell migration and invasion number, cyclin D1, MMP2 and MMP9 protein levels were apparently declined in miR-144-3p group (p<0.05), but cell proportion was elevated in G0/G1 phase.
| Group | miR-144-3p | A value | G0/G1 (%) | S (%) | G2 (%) | Cyclin D1 | MMP2 | MMP9 | Number of cell migration | Number of cell invasion |
|---|---|---|---|---|---|---|---|---|---|---|
| miR-con | 1.00±0.10 | 1.058±0.10 | 45.02±3.91 | 29.88±2.35 | 25.10±2.15 | 0.79±0.07 | 0.81±0.08 | 0.74±0.07 | 231±17.03 | 172±11.68 |
| miR-144-3p | 2.13±0.16* | 0.543±0.05* | 61.53±4.52* | 13.44±1.09* | 25.03±2.27 | 0.36±0.03* | 0.42±0.04* | 0.32±0.03* | 124±10.03* | 83±7.15* |
| t | 17.967 | 13.819 | 8.287 | 19.039 | 0.067 | 16.939 | 13.081 | 16.545 | 16.242 | 19.497 |
| P | 0.000 | 0.000 | 0.000 | 0.000 | 0.947 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05, relative to miR-control group
Table 4: miR-144-3P-Mediated Influence On SKOV3 Cell Malignant Behaviors (x̄±s, n=9)
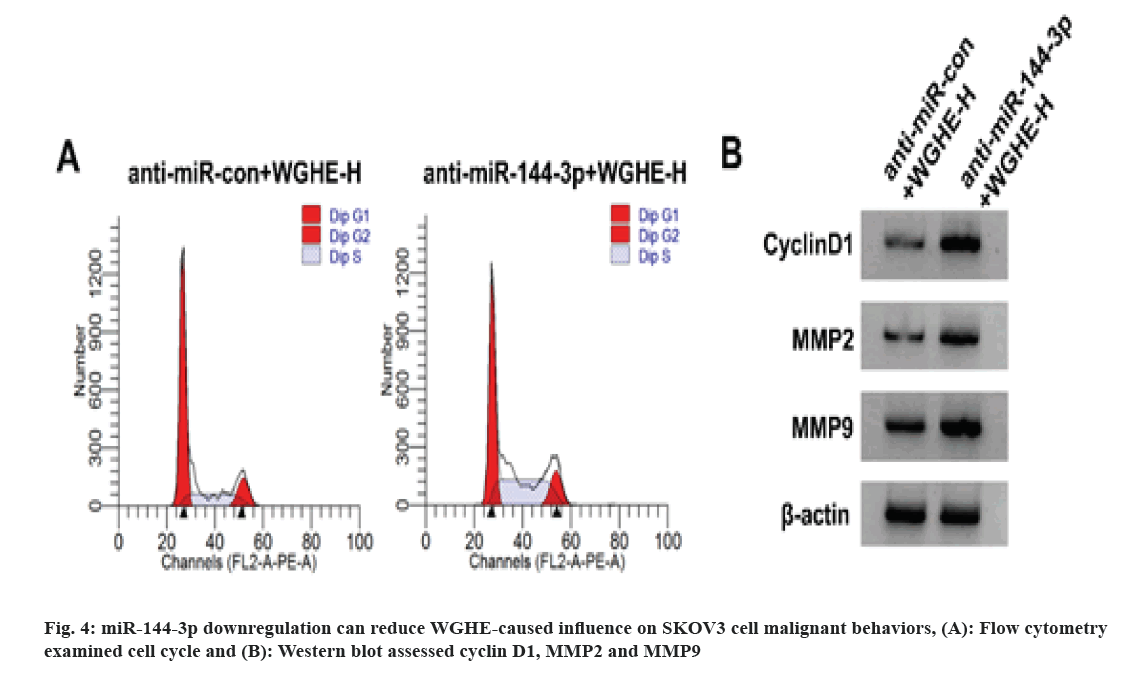
According to the results displayed in fig. 4 and Table 5, in comparison with the anti-miR-con+WGHE-H group, SKOV3 cell viability, cell proportion in S phase, SKOV3 cell migration and invasion and cyclin D1, MMP2 and MMP9 protein content were reduced in the miR-144-3p+WGHE-H group (p<0.05), but cell proportion was improved in G0/G1 phase (p<0.05).
| Group | miR-144-3p | A value | G0/G1 (%) | S (%) | G2 (%) | Cyclin D1 | MMP2 | MMP9 | Number of cell migration | Number of cell invasion |
|---|---|---|---|---|---|---|---|---|---|---|
| Anti-miR-con+WGHE-H | 1.00±0.10 | 0.448±0.04 | 62.13±5.14 | 14.06±1.15 | 23.81±2.14 | 0.42±0.03 | 0.38±0.03 | 0.32±0.03 | 103±8.16 | 73±5.17 |
| Anti-miR-144-3p+WGHE-H | 0.45±0.04 | 1.102±0.10* | 43.06±3.76* | 33.21±2.76* | 23.73±1.89 | 0.81±0.07* | 0.89±0.07* | 0.73±0.06* | 254±18.10* | 171±11.52* |
| t | 15.32 | 18.217 | 8.983 | 19.214 | 0.084 | 15.363 | 20.09 | 18.336 | 22.816 | 23.284 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.934 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05, relative to anti-miR-con+WGHE-H group
Table 5: Low Expression of miR-144-3P Can Reduce the Influence of WGHE on SKOV3 Cell Cycle, Migration and Invasion (x̄±s, n=9)
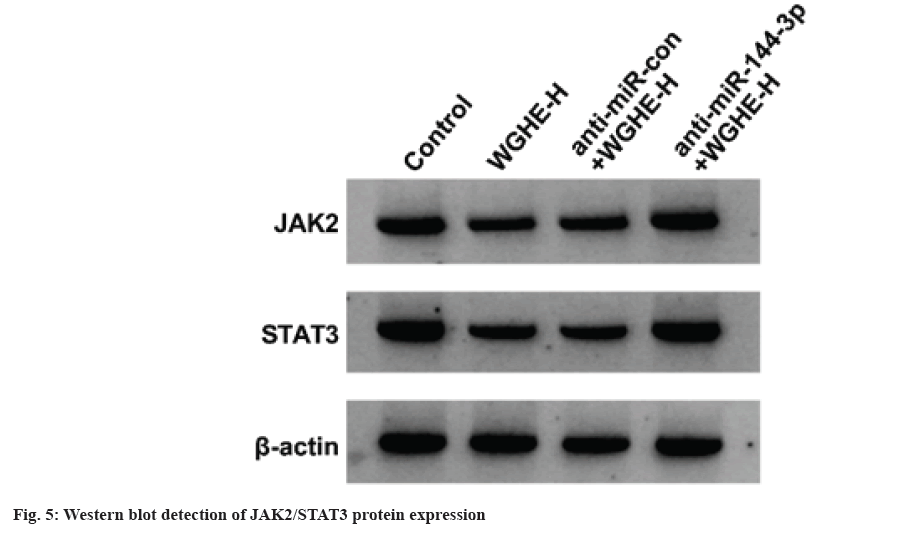
Compared with control group, JAK2 and STAT3 protein content was reduced in WGHE-H group (p<0.05), and JAK2 and STAT3 levels were increased in anti-miR-144-3p+WGHE-H group vs. anti-miR-con+WGHE-H group (p<0.05) as shown in fig. 5 and Table 6.
| Group | JAK2 | STAT3 |
|---|---|---|
| Control | 0.86±0.07 | 0.76±0.07 |
| WGHE-H | 0.42±0.04* | 0.38±0.03* |
| Anti-miR-con+WGHE-H | 0.40±0.03 | 0.36±0.04 |
| Anti-miR-144-3p+WGHE-H | 0.91±0.09# | 0.82±0.08# |
| F | 175.8 | 155.13 |
| p | 0.000 | 0.000 |
Note: *p<0.05 and #p<0.0, relative to control group and anti-miR-con+WGHE-H
Table 6: Western BLOT Detection of JAK2/STAT3 Protein Expression (x̄±s, n=9)
As a common gynecological malignancy with a high mortality rate, ovarian cancer still lacks effective treatment methods and often has a poor prognosis[11]. Notably, owing to the unique advantages of anti-tumor treatment, TCM may provide a new idea for the maintenance treatment of ovarian cancer[12]. Relevant literature has displayed that WGHE might suppress the invasiveness of prostate cancer cells[13]. Beyond that, WGHE might decrease esophageal cancer cell viability and cell cycle progression through different pathways[14]. Besides, WGHE was previously demonstrated to trigger cell apoptosis and curb breast cancer growth in the mouse xenograft model in vivo[15]. Herein, after treatment with different concentrations of WGHE, the SKOV3 cell activity, cycle progression, migration, invasion and cyclin D1, MMP2 and MMP9 contents were decreased. The above findings indicated WGHE could repress SKOV3 cell malignant behaviors.
Studies have delineated that miR-144-3p is down-regulated in cervical cancer cells and tissues, and overexpression of miR-144-3p can inhibit the growth and metastasis of cervical cancer cells[16]. Furthermore, miR-144-3p is lowly expressed in ovarian cancer tissues and cells, and reduced lncRNA HCG11 inhibits tumor development via regulating miR-144-3p[10]. After overexpression of miR-144-3p in this experiment, the activity of SKOV3 cells was decreased, cycle progression, migration and invasion were blocked, and cyclin D1, MMP2 and MMP9 contents were reduced. Increased miR-144-3p could inhibit SKOV3 cell proliferation, migration, invasion and cell cycle progression. This study found that WGHE treatment could increase miR-144-3p, which could be reversed via miR-144-3p inhibition in SKOV3 cells, indicating that WGHE may affect the progression of SKOV3 by regulating miR-144-3p.
JAK2/STAT3 is an important component of the expressionJAK/STAT pathway and its continuous activation in tumors has a cancer-promoting effect, which is expected to become a new anticancer drug target[17,18]. LncRNA Taurine-Upregulated Gene 1 (TUG1) interacts with miR-144 to promote hepatocellular carcinoma cell proliferation and migration via activating JAK2/STAT3 pathway[19]. Tilianin can promote the apoptosis of ovarian cancer cells and inhibit proliferation via the activity of JAK2/STAT3 signaling[20]. Here, JAK2 and STAT3 protein levels decreased after treatment with high-dose WGHE, indicating that WGHE restrained the activation of JAK2/STAT3 pathway. miR-144-3p inhibition could reverse WGHE-mediated influence on JAK2 and STAT3 protein expression, indicating that low miR-144-3p expression abrogated WGHE-triggered effect on JAK2/STAT3 pathway.
Taken together, WGHE inhibited the proliferation, migration and invasion of ovarian cancer cells by regulating the miR-144-3p/JAK2/STAT3 pathway.
Funding:
The work was supported by State Key Laboratory of Radiation Medicine and Protection (GZK1202140).
Conflict of interests:
The authors declared no conflict of interests.
References- Kim SI, Kim JW. Role of surgery and hyperthermic intraperitoneal chemotherapy in ovarian cancer. ESMO Open 2021;6(3):100149.
[Crossref] [Google Scholar] [PubMed]
- Wei J, Liu Z, He J, Liu Q, Lu Y, He S, et al. Traditional Chinese medicine reverses cancer multidrug resistance and its mechanism. Clin Transl Oncol 2022:24(3):471-82.
[Crossref] [Google Scholar] [PubMed]
- Wang R, Sun Q, Wang F, Liu Y, Li X, Chen T, et al. Efficacy and safety of Chinese herbal medicine on ovarian cancer after reduction surgery and adjuvant chemotherapy: A systematic review and meta-analysis. Front Oncol 2019;9:730.
[Crossref] [Google Scholar] [PubMed]
- Barekat S, Nasirpour A, Keramat J, Dinari M, Meziane-Kaci M, Paris C, et al. Phytochemical composition, antimicrobial, anticancer properties and antioxidant potential of green husk from several walnut varieties (Juglans regia L.). Antioxidants 2022;12(1):52.
- Xi M, Hou Y, Cai Y, Shen H, Ao J, Li M, et al. Antioxidant and antimicrobial characteristics of ethyl acetate polar fractions from walnut green husk. J Food Sci 2023;88(3):1060-74.
[Crossref] [Google Scholar] [PubMed]
- Zhang J, Zhang J, Zhao C, Sui H, feng Li C, Zhong L, et al. Green walnut husk extracts proliferation and migration in gastric cancer. J Cancer 2022;13(4):1130-44.
[Crossref] [Google Scholar] [PubMed]
- Alshatwi AA, Hasan TN, Shafi G, Syed NA, Al-Assaf AH, Alamri MS, et al. Validation of the antiproliferative effects of organic extracts from the green husk of Juglans regia L. on PC-3 human prostate cancer cells by assessment of apoptosis-related genes. Evid Based Complement Altern Med 2012;2012:103026.
[Crossref] [Google Scholar] [PubMed]
- Zhou GY, Yi YX, Jin LX, Lin W, Fang PP, Lin XZ, et al. The protective effect of juglanin on fructose-induced hepatitis by inhibiting inflammation and apoptosis through TLR4 and JAK2/STAT3 signaling pathways in fructose-fed rats. Biomed Pharmacother 2016;81:318-28.
[Crossref] [Google Scholar] [PubMed]
- Yuan D, Guo T, Qian H, Ge H, Zhao Y, Huang A, et al. Icariside II suppresses the tumorigenesis and development of ovarian cancer by regulating miR-144-3p/IGF2R axis. Drug Dev Res 2022;83(6):1383-93.
[Crossref] [Google Scholar] [PubMed]
- Li XF, Hu DM, Zhao YX, Zhang L, Jin Y. Knockdown of lncRNA HCG11 suppresses cell progression in ovarian cancer by modulating miR-144-3p/PBX3. Eur Rev Med Pharmacol Sci 2020;24(21):11032-40.
[Crossref] [Google Scholar] [PubMed]
- Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Barroilhet L, Behbakht K, Berchuck A, et al. Ovarian cancer, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2021;19(2):191-226.
[Crossref] [Google Scholar] [PubMed]
- Dou L, Zou D, Song F, Jin Y, Li Y, Zhang Y. Bufalin suppresses ovarian cancer cell proliferation via EGFR pathway. Chin Med J 2022;135(4):456-61.
[Crossref] [Google Scholar] [PubMed]
- Fang F, Chen S, Ma J, Cui J, Li Q, Meng G, et al. Juglone suppresses epithelial-mesenchymal transition in prostate cancer cells via the protein kinase B/glycogen synthase kinase-3ß/Snail signaling pathway. Oncol Lett 2018;16(2):2579-84.
[Crossref] [Google Scholar] [PubMed]
- Li C, Zhang Z, Zhang S, Yan W, Si C, Lee MH, et al. Inhibitory effects of the extracts of Juglans sigillata green husks on the proliferation, migration and survival of KYSE150 and EC9706 human esophageal cancer cell lines. Nutr Cancer 2019;71(1):149-58.
[Crossref] [Google Scholar] [PubMed]
- Sun ZL, Dong JL, Wu J. Juglanin induces apoptosis and autophagy in human breast cancer progression via ROS/JNK promotion. Biomed Pharmacother 2017;85:303-12.
[Crossref] [Google Scholar] [PubMed]
- Wu J, Zhao Y, Li F, Qiao B. miR-144-3p: A novel tumor suppressor targeting MAPK6 in cervical cancer. J Physiol Biochem 2019;75(2):143-52.
- Thomas SJ, Snowden JA, Zeidler MP, Danson SJ. The role of JAK/STAT signalling in the pathogenesis, prognosis and treatment of solid tumours. Br J Cancer 2015;113(3):365-71.
[Crossref] [Google Scholar] [PubMed]
- Yoshikawa T, Miyamoto M, Aoyama T, Soyama H, Goto T, Hirata J, et al. JAK2/STAT3 pathway as a therapeutic target in ovarian cancers. Oncol Lett 2018;15(4):5772-80.
[Crossref] [Google Scholar] [PubMed]
- Lv J, Kong Y, Gao Z, Liu Y, Zhu P, Yu Z. LncRNA TUG1 interacting with miR-144 contributes to proliferation, migration and tumorigenesis through activating the JAK2/STAT3 pathway in hepatocellular carcinoma. Int J Biochem Cell Biol 2018;101:19-28.
[Crossref] [Google Scholar] [PubMed]
- Xiong C, Yan B, Xia S, Yu F, Zhao J, Bai H. Tilianin inhibits the human ovarian cancer (PA-1) cell proliferation via blocking cell cycle, inducing apoptosis and inhibiting JAK2/STAT3 signaling pathway. Saudi J Biol Sci 2021;28(9):4900-7.
[Crossref] [Google Scholar] [PubMed]
- Kim SI, Kim JW. Role of surgery and hyperthermic intraperitoneal chemotherapy in ovarian cancer. ESMO Open 2021;6(3):100149.
[Crossref] [Google Scholar] [PubMed]
- Wei J, Liu Z, He J, Liu Q, Lu Y, He S, et al. Traditional Chinese medicine reverses cancer multidrug resistance and its mechanism. Clin Transl Oncol 2022:24(3):471-82.
[Crossref] [Google Scholar] [PubMed]
- Wang R, Sun Q, Wang F, Liu Y, Li X, Chen T, et al. Efficacy and safety of Chinese herbal medicine on ovarian cancer after reduction surgery and adjuvant chemotherapy: A systematic review and meta-analysis. Front Oncol 2019;9:730.
[Crossref] [Google Scholar] [PubMed]
- Barekat S, Nasirpour A, Keramat J, Dinari M, Meziane-Kaci M, Paris C, et al. Phytochemical composition, antimicrobial, anticancer properties and antioxidant potential of green husk from several walnut varieties (Juglans regia L.). Antioxidants 2022;12(1):52.
- Xi M, Hou Y, Cai Y, Shen H, Ao J, Li M, et al. Antioxidant and antimicrobial characteristics of ethyl acetate polar fractions from walnut green husk. J Food Sci 2023;88(3):1060-74.
[Crossref] [Google Scholar] [PubMed]
- Zhang J, Zhang J, Zhao C, Sui H, feng Li C, Zhong L, et al. Green walnut husk extracts proliferation and migration in gastric cancer. J Cancer 2022;13(4):1130-44.
[Crossref] [Google Scholar] [PubMed]
- Alshatwi AA, Hasan TN, Shafi G, Syed NA, Al-Assaf AH, Alamri MS, et al. Validation of the antiproliferative effects of organic extracts from the green husk of Juglans regia L. on PC-3 human prostate cancer cells by assessment of apoptosis-related genes. Evid Based Complement Altern Med 2012;2012:103026.
[Crossref] [Google Scholar] [PubMed]
- Zhou GY, Yi YX, Jin LX, Lin W, Fang PP, Lin XZ, et al. The protective effect of juglanin on fructose-induced hepatitis by inhibiting inflammation and apoptosis through TLR4 and JAK2/STAT3 signaling pathways in fructose-fed rats. Biomed Pharmacother 2016;81:318-28.
[Crossref] [Google Scholar] [PubMed]
- Yuan D, Guo T, Qian H, Ge H, Zhao Y, Huang A, et al. Icariside II suppresses the tumorigenesis and development of ovarian cancer by regulating miR-144-3p/IGF2R axis. Drug Dev Res 2022;83(6):1383-93.
[Crossref] [Google Scholar] [PubMed]
- Li XF, Hu DM, Zhao YX, Zhang L, Jin Y. Knockdown of lncRNA HCG11 suppresses cell progression in ovarian cancer by modulating miR-144-3p/PBX3. Eur Rev Med Pharmacol Sci 2020;24(21):11032-40.
[Crossref] [Google Scholar] [PubMed]
- Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Barroilhet L, Behbakht K, Berchuck A, et al. Ovarian cancer, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2021;19(2):191-226.
[Crossref] [Google Scholar] [PubMed]
- Dou L, Zou D, Song F, Jin Y, Li Y, Zhang Y. Bufalin suppresses ovarian cancer cell proliferation via EGFR pathway. Chin Med J 2022;135(4):456-61.
[Crossref] [Google Scholar] [PubMed]
- Fang F, Chen S, Ma J, Cui J, Li Q, Meng G, et al. Juglone suppresses epithelial-mesenchymal transition in prostate cancer cells via the protein kinase B/glycogen synthase kinase-3ß/Snail signaling pathway. Oncol Lett 2018;16(2):2579-84.
[Crossref] [Google Scholar] [PubMed]
- Li C, Zhang Z, Zhang S, Yan W, Si C, Lee MH, et al. Inhibitory effects of the extracts of Juglans sigillata green husks on the proliferation, migration and survival of KYSE150 and EC9706 human esophageal cancer cell lines. Nutr Cancer 2019;71(1):149-58.
[Crossref] [Google Scholar] [PubMed]
- Sun ZL, Dong JL, Wu J. Juglanin induces apoptosis and autophagy in human breast cancer progression via ROS/JNK promotion. Biomed Pharmacother 2017;85:303-12.
[Crossref] [Google Scholar] [PubMed]
- Wu J, Zhao Y, Li F, Qiao B. miR-144-3p: A novel tumor suppressor targeting MAPK6 in cervical cancer. J Physiol Biochem 2019;75(2):143-52.
- Thomas SJ, Snowden JA, Zeidler MP, Danson SJ. The role of JAK/STAT signalling in the pathogenesis, prognosis and treatment of solid tumours. Br J Cancer 2015;113(3):365-71.
[Crossref] [Google Scholar] [PubMed]
- Yoshikawa T, Miyamoto M, Aoyama T, Soyama H, Goto T, Hirata J, et al. JAK2/STAT3 pathway as a therapeutic target in ovarian cancers. Oncol Lett 2018;15(4):5772-80.
[Crossref] [Google Scholar] [PubMed]
- Lv J, Kong Y, Gao Z, Liu Y, Zhu P, Yu Z. LncRNA TUG1 interacting with miR-144 contributes to proliferation, migration and tumorigenesis through activating the JAK2/STAT3 pathway in hepatocellular carcinoma. Int J Biochem Cell Biol 2018;101:19-28.
[Crossref] [Google Scholar] [PubMed]
- Xiong C, Yan B, Xia S, Yu F, Zhao J, Bai H. Tilianin inhibits the human ovarian cancer (PA-1) cell proliferation via blocking cell cycle, inducing apoptosis and inhibiting JAK2/STAT3 signaling pathway. Saudi J Biol Sci 2021;28(9):4900-7.
[Crossref] [Google Scholar] [PubMed]
- Kim SI, Kim JW. Role of surgery and hyperthermic intraperitoneal chemotherapy in ovarian cancer. ESMO Open 2021;6(3):100149.
[Crossref] [Google Scholar] [PubMed]
- Wei J, Liu Z, He J, Liu Q, Lu Y, He S, et al. Traditional Chinese medicine reverses cancer multidrug resistance and its mechanism. Clin Transl Oncol 2022:24(3):471-82.
[Crossref] [Google Scholar] [PubMed]
- Wang R, Sun Q, Wang F, Liu Y, Li X, Chen T, et al. Efficacy and safety of Chinese herbal medicine on ovarian cancer after reduction surgery and adjuvant chemotherapy: A systematic review and meta-analysis. Front Oncol 2019;9:730.
[Crossref] [Google Scholar] [PubMed]
- Barekat S, Nasirpour A, Keramat J, Dinari M, Meziane-Kaci M, Paris C, et al. Phytochemical composition, antimicrobial, anticancer properties and antioxidant potential of green husk from several walnut varieties (Juglans regia L.). Antioxidants 2022;12(1):52.
- Xi M, Hou Y, Cai Y, Shen H, Ao J, Li M, et al. Antioxidant and antimicrobial characteristics of ethyl acetate polar fractions from walnut green husk. J Food Sci 2023;88(3):1060-74.
[Crossref] [Google Scholar] [PubMed]
- Zhang J, Zhang J, Zhao C, Sui H, feng Li C, Zhong L, et al. Green walnut husk extracts proliferation and migration in gastric cancer. J Cancer 2022;13(4):1130-44.
[Crossref] [Google Scholar] [PubMed]
- Alshatwi AA, Hasan TN, Shafi G, Syed NA, Al-Assaf AH, Alamri MS, et al. Validation of the antiproliferative effects of organic extracts from the green husk of Juglans regia L. on PC-3 human prostate cancer cells by assessment of apoptosis-related genes. Evid Based Complement Altern Med 2012;2012:103026.
[Crossref] [Google Scholar] [PubMed]
- Zhou GY, Yi YX, Jin LX, Lin W, Fang PP, Lin XZ, et al. The protective effect of juglanin on fructose-induced hepatitis by inhibiting inflammation and apoptosis through TLR4 and JAK2/STAT3 signaling pathways in fructose-fed rats. Biomed Pharmacother 2016;81:318-28.
[Crossref] [Google Scholar] [PubMed]
- Yuan D, Guo T, Qian H, Ge H, Zhao Y, Huang A, et al. Icariside II suppresses the tumorigenesis and development of ovarian cancer by regulating miR-144-3p/IGF2R axis. Drug Dev Res 2022;83(6):1383-93.
[Crossref] [Google Scholar] [PubMed]
- Li XF, Hu DM, Zhao YX, Zhang L, Jin Y. Knockdown of lncRNA HCG11 suppresses cell progression in ovarian cancer by modulating miR-144-3p/PBX3. Eur Rev Med Pharmacol Sci 2020;24(21):11032-40.
[Crossref] [Google Scholar] [PubMed]
- Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Barroilhet L, Behbakht K, Berchuck A, et al. Ovarian cancer, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2021;19(2):191-226.
[Crossref] [Google Scholar] [PubMed]
- Dou L, Zou D, Song F, Jin Y, Li Y, Zhang Y. Bufalin suppresses ovarian cancer cell proliferation via EGFR pathway. Chin Med J 2022;135(4):456-61.
[Crossref] [Google Scholar] [PubMed]
- Fang F, Chen S, Ma J, Cui J, Li Q, Meng G, et al. Juglone suppresses epithelial-mesenchymal transition in prostate cancer cells via the protein kinase B/glycogen synthase kinase-3ß/Snail signaling pathway. Oncol Lett 2018;16(2):2579-84.
[Crossref] [Google Scholar] [PubMed]
- Li C, Zhang Z, Zhang S, Yan W, Si C, Lee MH, et al. Inhibitory effects of the extracts of Juglans sigillata green husks on the proliferation, migration and survival of KYSE150 and EC9706 human esophageal cancer cell lines. Nutr Cancer 2019;71(1):149-58.
[Crossref] [Google Scholar] [PubMed]
- Sun ZL, Dong JL, Wu J. Juglanin induces apoptosis and autophagy in human breast cancer progression via ROS/JNK promotion. Biomed Pharmacother 2017;85:303-12.
[Crossref] [Google Scholar] [PubMed]
- Wu J, Zhao Y, Li F, Qiao B. miR-144-3p: A novel tumor suppressor targeting MAPK6 in cervical cancer. J Physiol Biochem 2019;75(2):143-52.
- Thomas SJ, Snowden JA, Zeidler MP, Danson SJ. The role of JAK/STAT signalling in the pathogenesis, prognosis and treatment of solid tumours. Br J Cancer 2015;113(3):365-71.
[Crossref] [Google Scholar] [PubMed]
- Yoshikawa T, Miyamoto M, Aoyama T, Soyama H, Goto T, Hirata J, et al. JAK2/STAT3 pathway as a therapeutic target in ovarian cancers. Oncol Lett 2018;15(4):5772-80.
[Crossref] [Google Scholar] [PubMed]
- Lv J, Kong Y, Gao Z, Liu Y, Zhu P, Yu Z. LncRNA TUG1 interacting with miR-144 contributes to proliferation, migration and tumorigenesis through activating the JAK2/STAT3 pathway in hepatocellular carcinoma. Int J Biochem Cell Biol 2018;101:19-28.
[Crossref] [Google Scholar] [PubMed]
- Xiong C, Yan B, Xia S, Yu F, Zhao J, Bai H. Tilianin inhibits the human ovarian cancer (PA-1) cell proliferation via blocking cell cycle, inducing apoptosis and inhibiting JAK2/STAT3 signaling pathway. Saudi J Biol Sci 2021;28(9):4900-7.
[Crossref] [Google Scholar] [PubMed]