- *Corresponding Author:
- B. Vuyyala
Department of Pharmacology, School of Pharmacy, Gurunanak Institutions, Ibrahimpatnam, Telangana 501506, India
E-mail: balakrishnavuyyala@gmail.com
| Date of Received | 07 September 2021 |
| Date of Revision | 08 October 2022 |
| Date of Acceptance | 16 November 2022 |
| Indian J Pharm Sci 2022;84(6):1453-1462 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Tamarindus indica (Fabaceae) have been using in traditional medicine to cure various diseases, including mental stress and anxiousness. The current study was aimed to evaluate phytochemical analysis and anxiolytic activity of Tamarindus indica flower extracts (n-Hexane, Ethyl acetate, Chloroform and methanol) using different animal models such as elevated plus-maze, mirror chamber, light/dark, hole board tests. The extracts had showed presence of different phytochemical constituents. The mice were given two different doses of these extracts to see whether they had any anti-anxiety effects in an elevated maze-plus experiment. The methanol extract showed prominent activity compared to other extracts. The methanol extract was used for further analysis using column chromatography and four fractions (F1-F4) were separated and their activity was tested and further to investigate the anti-anxiety properties. The fraction F3 had showed better anxiolytic activity and using preparative thin layer chromatography three fractions were isolated. The fraction (F3.2) was identified as flavonoid through phytochemical tests and Fourier transform infrared, Nuclear Magnetic Resonance, data as showed 3570 (-OH Str, Ar-OH) and 1753(C=O Str). The fraction F3.2 has anti-anxiety properties (4.8±0.31 and 7.2±0.30 secs) elevated by elevated plus maze test. Fraction F3.2 at 80 mg/kg dose administered orally showed a considerable anti-anxiety effect on different anxiolytic activity tests. From the results of current study, there is a need to do a thorough investigation in finding out the mechanism of action whether it is either through the serotonergic or GABAergic pathway of fraction F3.2. The current study was may be first report about the flavonoids had showing anti-anxiety properties of Tamarindus indica flower extracts.
Keywords
Anxiety, Tamarindus indica flower, flavonoids, fraction F3.2, elevated plus-maze
Anxiety is a normal feeling commonly present in every one’s daily life that comes and goes[1]. But in some people anxiety became as emotional disorder such as panic, phobia, social anxiety, obsessive compulsive disorder, illness anxiety disorder etc., These disorders can affect anyone due to different causes like stress, health problems, living environmental conditions[2]. The researchers believe that some part of the brain responsible in controlling the anxiety related risk factors such as shyness, nervousness, negative feeling, confusion etc. Recent studies reveal, one-eighth worlds’ population are suffering with Anxiety Disorder (AD)[3-5]. The ADs became more interesting research in psychopharmacology research. There were different categories to control the ADs includes psychotherapy, complemental health techniques and medications[6-8]. The psychotherapy and complemental health techniques are natural therapies i.e., their treatment by cognitive behavioral therapy, exposure to different reposes, yoga, mindfulness, self-management strategies[9]. But, treatment with medication includes different medicines (drugs) depends on types of ADs such as serotonin uptake inhibitors, norepinephrine reuptake inhibitors, anti-psychotics, benzodiazepines[10]. The regular use of different medications is also causing various side effects. In recent time, the people are turning back to natural medication i.e., herbal medicine to treat diseases because of their easy availability, low cost and less side effects[11].
Herbal Medicine (HM) has been using since older days in different parts of world to treat various diseases. Medicinal plants are mainly use in HM and there were very little written evidences available on usage of different medicinal plants about their medicinal use[12,13]. In recent decades, upon interest on HM researchers actively reporting medicinal uses of various plants scientifically and identifying broad spectrum biologically active compounds to treat different diseases including ADs[14-17]. Tamarindus indica (T. indica) is one of such medicinal plant have different medical uses such as for treatment of stomach ailments such as abdominal pain, diarrhea and constipation and reported to have laxative, anti-microbial, anti-parasitic, anti-fungal, anti-viral and anti-nematodal properties[18-21]. The researchers around the world have been reported various medicinal properties and bioactive compounds from different parts of T. indica[22,23]. But there was very little research work reported on phytochemical constituents and biological activities of T. indica flowers. So, the current study aimed to characterize the Phyto-constituents and evaluation of anxiolytic activity of T. indica flower extracts.
Materials and Methods
Drugs and reagents:
The reagents used in current study were analytical grade. Capillary tubes, Thin Layer Chromatography (TLC) plates were obtained from Merck, Germany. Diazepam was from Jawa Pharmaceuticals Pvt. Ltd., Gurgaon. Carboxy methyl cellulose from Bioven Ingredients, Tween 80 from Labogen’s Fine Chem Industry.
Collection of plant material and preparation of extracts:
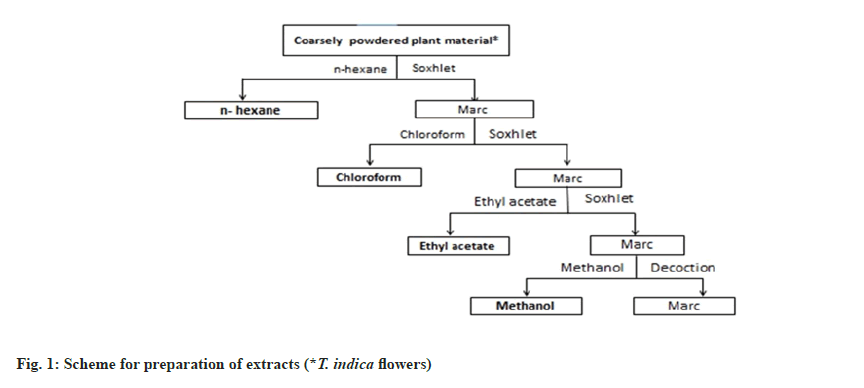
The flowers of T. indica were collected around Hyderabad region, Telangana. The collected flower material was shade dried and made into coarse powder. The coarse powder was used for extraction through Soxhlation using hexane, chloroform, ethyl acetate and methanol serially as shown in fig. 1. The retrieved solvents were evaporated utilizing a rotary vacuum evaporator under pressure. The obtained extracts were stored in a desiccator for further usage.
Experimental animals:
Swiss albino mice of either sex weighing 25±5 g around 6 to 8 w age for anxiolytic activity and female rats weighing 180-220 g with age between 8 to 12 w for acute toxicity study were used in the study. The animals were maintained at 23°±4° with 66±4 % relative humidity with light/dark cycles at the animal house, Guru Nanak Institution Technical Campus-School of Pharmacy, Khanapur and arranged standard nutrition and free access to water. Before initiation of anxiolytic activity, the extracts were tested for their toxicity. The use and care of animals in the current study, the protocol was approved by the Ethics Committee of the Institute for animals' usage, Guru Nanak Institutions Technical Campus (GNITC)-School of Pharmacy (Authorization No: 06/GNIP/CPCSEA/IAEC/2019).
Preparation of dosages:
Carboxy methyl cellulose (0.5 % w/v) with 5 % tween (80) has been utilized as a vehicle to prepare the test dosages of various extracts and standard drug diazepam. The vehicle acts as control. The extracts/fractions/ fraction F3.2 (BVT1) and diazepam at 20, 40, 60, 80, 100 mg/kg and 2 mg/kg were prepared and through the peroral route with a tuberculin syringe using a cannula (oral) were administrated.
Acute oral toxicity studies:
The acute oral toxicity of T. indica flower extracts was carried as per Organization for Economic Co-operation and Development (OECD) main test 423. Before initiation of the study animals were acclimatized to laboratory condition and undergone overnight fasting. The rats were divided into four groups (n=4) for each extract of T. indica and were administrated at a dose of 5, 50, 300 and 2000 mg/kg body weight (b. w). The animals were under observation up to 14 d to notice any physio-psychological changes and finally lethal conditions[24,25].
Phytochemical analysis:
The T. indica flower extracts were analyzed to explore their phytochemical profile using standard phytochemical tests[1].
Anxiolytic activity:
Elevated Plus-Maze (EPM) Model: EPM model have been widely using method to assess anxiety behavior in animals. An EPM with two open (16×5 cm) and two closed arms (16×5×12 cm) with an open roof keep elevating (25 cm) above the floor was used. Before the experiments, mice are permitted to interact (socialize) during the trial. Other than the maze's height, every measure is taken to guarantee that no external stimuli might cause stress in animals. A dosage plan is set up; each individual mouse gets it. Activate EPM 60 min after receiving the vehicle, test material, or diazepam treatment. Each individual is placed at the midpoint of EPM and made sure that it is faced towards the open arm. The mice's behavior during a 5 min trial is recorded as follows in open arms: Total entries and time spent by mice[26].
Mirror chamber test: Open on one side, the mirror chamber equipment comprised a 30 cm mirror cube put within a 40×40×30.5 cm wooden box to create a 5 cm corridor that entirely encircled the chamber used. On the wooden box wall, a mirror pointing towards the open side of the mirror compartment was placed. The box was colored entirely in black on rest three sides. After a 1 h dosage delivery period, individual mice were put in a corridor at a specified corner. For the duration of the 5 min test, the below data was collected in a mirror chamber; latency for entering, total entries and time spent[27,28].
Light/dark test: The light/dark equipment with rectangular shaped box (46×27×30 cm) is parted into two areas, one being small and the other large (18×27 cm) and (27×27 cm), respectively. In the middle of the floor separation, an opening door (7.5×7.5 cm) was positioned. Unlike the compartment of the small area, which was coated with black color, the large compartment was coated with white color and brilliantly lighted by a sixty-watt cold light source. Each mouse was separately put in the middle of the light chamber 1 h after the test medication was administered (facing opposite the door). The duration spent in the light zone and the delay for the initial passage from light to darkroom were measured during a 5 min test session[29].
Hole board test: The test equipment was made out of a 40×40 cm Perspex panel with sixteen holes separated by equal distance on the floor[30]. The panel was 15 cm above the surface of the table. Head dips were noted during a 5 min phase in which each animal was put in the middle of the plank, facing opposite from the viewer.
Sample preparation for column chromatography:
The results of phytochemical screening reveals, presence of variation in phytoconstituents in different extracts of T. indica flower. Based on results of anxiolytic activity and availability of extracts, methanol extract of T. indica flower was used to isolate bioactive molecules. The methanol extract of T. indica weighed ≈200 gm was dissolved in methanol:water (50:50 v/v) and extracted with ethyl acetate 1:1 ratio. The collected ethyl acetate fractions were combined. 75 g of silica powder was dissolved in the aforementioned ethyl acetate solution for surface coating. Afterwards, it was dried and stored in a vacuum desiccator before being exposed to vacuum evaporation in an Eyela N 1100 rotary evaporator at 110°. The resulting silica powder was used further for the column chromatography.
Column chromatography:
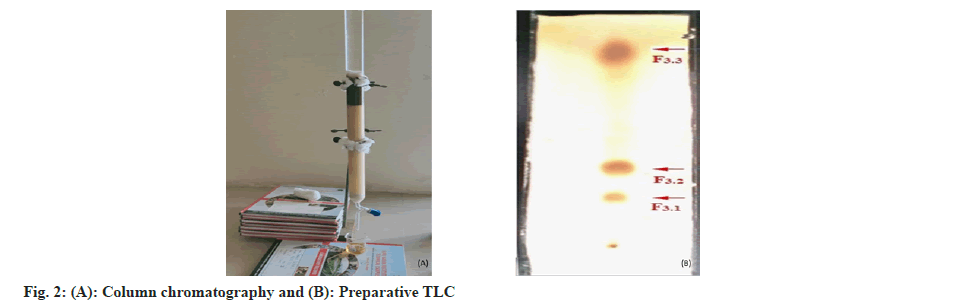
The prepared extract was used for column chromatography (2.5×75 cm) with silica gel of particle size 250-410 µm. Silica gel (150 g) and chloroform were mixed together to make a slurry, which was then used to load the column[31] as showed in fig. 2A. The superficial layer of column is coated silica powder for phytoconstituent segregation and the column was evaded with chloroform, 400 ml first, then ethyl acetate in chloroform, then methanol in ethyl acetate (0, 10, 20, 30, 40, 50, 52.5, 55, 57.5, 60, 62.5, 65, 67.5, 70, 72.5, 75, 77.5, 80, 90 and 100 %). For elution, used 200 ml of each solvent preparation and collected each fraction in 25 ml container, labeled and were evaporated in a rotary evaporator.
TLC and preparative TLC:
The collected fractions from column chromatography were used to identify different phytochemicals with n-hexane and methanol as mobile phases in various ratios. The charring solution i.e., 10 % sulfuric acid in methanol (v/v) was used to visualize the phyto-molecules. The comparable fractions were collected and mixed. The fractions 13-17 of column chromatography are used to separate phytoconstituents using preparative TLC (fig. 2B).
Statistical analysis:
The results in the current study were reported as mean±Standard Error of the Mean (mean±SEM) and performed the one-way analysis of variance with tukey’s test to compare the means of all treatments to the mean of every other treatment. A p-value under 0.05 is deemed significant in Tukey's test.
Results and Discussion
The dried power of T. indica flowers was used to prepare different extracts as showed in fig. 1 and obtained the different percentage w/w yields with different solvents. 1.86, 1.20, 8.38 and 18.44 are percentage yield to hexane, chloroform, ethyl acetate and methanol respectively. The prepared extracts were used to explore their phytochemicals with standard screening tests. The results were showed in Table 1. There was difference between all extracts were observed, all extracts gave negative results for glycosides, coumarins, proteins, amino acids. Hexane extract gave positive results to flavonoids, tannins. Chloroform extract gave positive results for alkaloids, steroids. Ethyl acetate extract gave positive results for saponins, carbohydrates. Methanol extract gave positive results for steroids, terpenoids, saponins, flavonoids, tannins, carbohydrates (Table 1).
| Phytoconstituents | Name of the extract | |||
|---|---|---|---|---|
| n-hexane | Chloroform | Ethyl acetate | Methanol | |
| Alkaloids | - | + | - | - |
| Glycosides | - | - | - | - |
| Steroids | - | + | - | + |
| Terpenoids | - | - | - | + |
| Saponins | - | - | + | + |
| Coumarins | - | - | - | - |
| Flavonoids | + | - | - | + |
| Tannins | + | - | - | + |
| Carbohydrates | - | - | + | + |
| Proteins | - | - | - | - |
| Amino acids | - | - | - | - |
Note: +=Presence; -=Absence
Table 1: Phytochemical Screening of various extracts of Tamarindus indicia flower.
Acute toxicity study:
The toxicity studies were conducted to all prepared extracts as per OECD guidelines. There were no abnormal, physio-psychological changes, mortality was observed for tested doses and the maximum dose 2000 mg/kg b.w.
Anxiolytic activity and column chromatography:
The prepared extracts were analyzed to evaluate the anti-anxiolytic activity using EPM and locomotor activities. The crude extracts were analyzed on dose basis at 200 and 400 mg/kg b. w. The extracts had showed dose dependent activity in controlling the anxiety and different extracts had showed different activeness as their variation in phytochemical constituents. In our previous reports, among four extracts methanol extract was showed more activity compared to other extracts and its results were comparable with standard drug diazepam with more entries and time spent in open arms. The hexane extract has showed less activity. The chloroform and ethyl acetate extract has showed moderate activities[32]. Based on previous report, methanol extract was used to isolate bioactive compounds using column chromatography (fig. 2A and 2B).
The column chromatography was prepared as described in materials and methods. The column was run with starting of 100 % chloroform and then with different combinations of ethyl acetate and methanol by increasing polarity. 200 ml of each fraction was collected and evaluated for their phytoconstituents. Then evaporated under vacuum using rotavapor and stored in desiccator for further use. The collected fractions were tested for phytochemical analysis using standard procedures. The collected fraction (13-17) gave positive results for flavonoids. The fractions were aggregated and observed for phytochemical separation using TLC with mobile phase methanol and n-hexane as 9:1 v/v. The TLC profile produced four fractions: F1-F4. Following was the yield (%) of the fractions: F1 (15.8), F2 (24.3), F3 (31.2) and F4 (28.6). The F3 was again separated through preparative TLC with mobile phase methanol in ethyl acetate and obtained F3.1 (28.6), F3.2 (34.4), and F3.3 (30.8) as fig. 2B.
The fractions obtained (F3.1 to F3.3) from methanol extracts were tested for their anxiolytic activity using EPM model at 20 and 40 mg/kg b.w. and dose-dependent strong anti-anxiety effect was seen in F3.2 (Table 2). Among all sub fractions, F3.2 demonstrated a maximum anti-anxiety activity with more entries and maximum time spent in open arms of the EPM i.e. 4.8±0.31 and 7.2±0.3 sec.
| Treatment | Dose (mg/kg) | Total entries in open arms (mean±SEM) | Time spent (sec) in open arms (mean±SEM) |
|---|---|---|---|
| Vehicle | 0.25 ml | 2.3±0.21b,# | 2.8±0.3b,# |
| Diazepam | 2 | 7.2±0.4a,# | 14.5±0.42a,# |
| F3.1 | 20 | 2.5±0.22b,# | 2.6±0.25b,# |
| F3.1 | 40 | 2.6±0.33b,# | 2.8±0.3b,# |
| F3.2 | 20 | 3.6±0.21a,#,b,# | 3.8±0.31a,b,# |
| F3.2 | 40 | 4.8±0.31a,#,b,$ | 7.2±0.3a,b,# |
| F3.3 | 20 | 2.8±0.30b,# | 2.5±0.22b,# |
| F3.3 | 40 | 3.1±0.31b,# | 2.6±0.33b,# |
Note: n=6, $: p<0.01, #: p<0.001, a: vs. control; b: vs. diazepam. Tukey’s multiple comparison test is used after a one-way Analysis Of Variance (ANOVA).
Table 2: Evaluation of Anti-Anxiety of F3.1 TO F3.3.
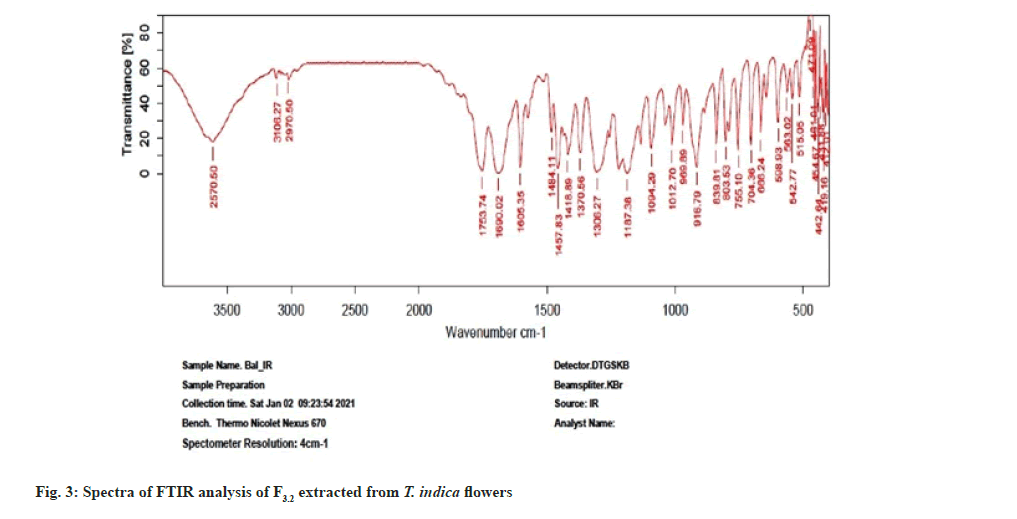
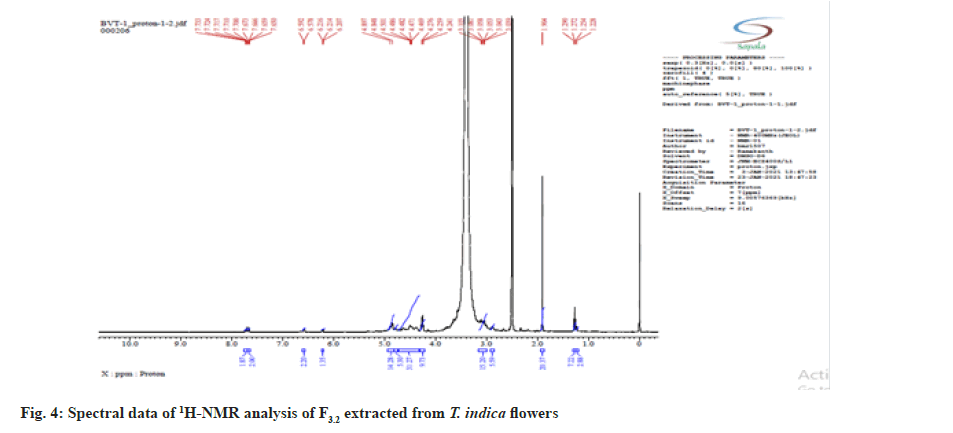
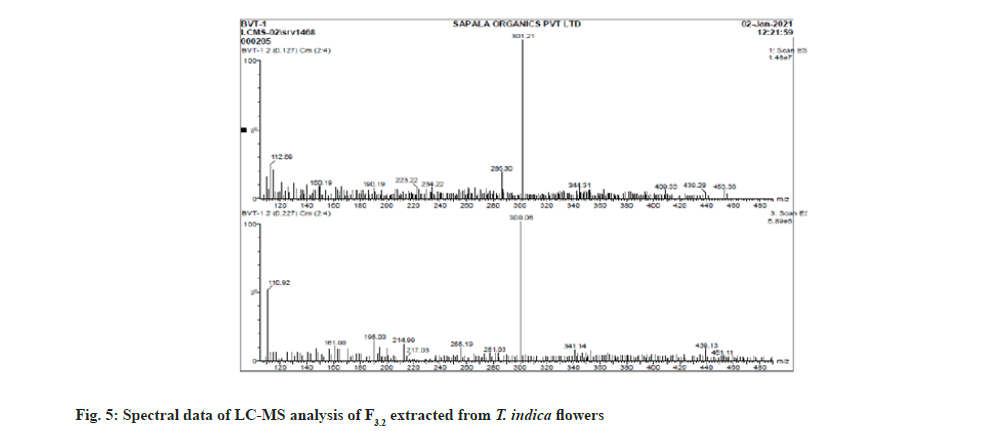
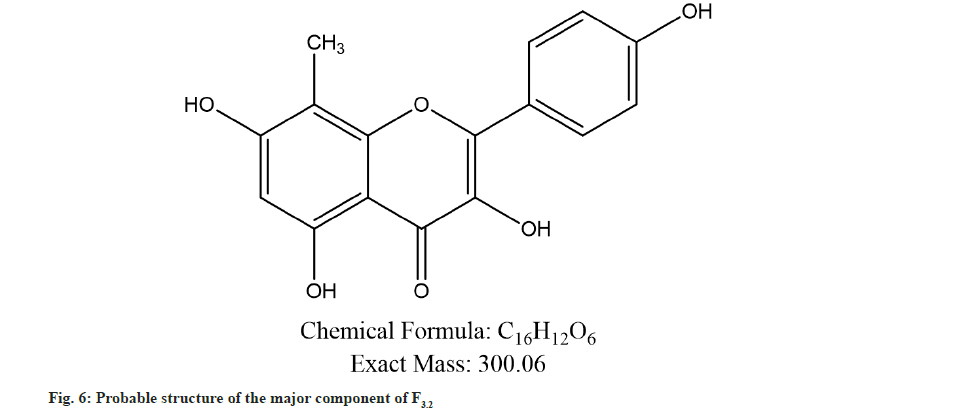
Further the F3.2 was evaluated for phytochemical screening with standard procedures and structural elucidations using Fourier Transform Infrared (FTIR) (Table 3 and fig. 3), 1H Nuclear Magnetic Resonance (NMR) (fig. 4), and Mass Spectra (MS) (fig. 5). The phytochemical analysis of F3.2 gave positive results for flavonoid and structural elucidation of the spectral data obtained from FTIR, NMR, MS demonstrates that the mass of the isolated compound is around 300.06 and structural formula is C16H12O6. The probable structure was showed in fig. 6 and named it as BVT1 (F3.2).
| Characterization studies | Result |
|---|---|
| Melting point | 253° |
| UV λmax (methanol) | 269 nm |
| FTIR spectroscopy(cm-1 KBr) | 3570 (-OH Str, Ar-OH), |
| 3106(-CH Str, Phenyl); 2970 (-CH Str, Alkane); | |
| 1753 (C=O Str); 1640 (C-C Str, Phenyl); | |
| 1484 (C=C Str, Phenyl); 1187 (C-O Bend) | |
| ESI MS (m/z) | C16H12O6-300.06, [M+H]- ion at 301.02 |
Note: FTIR: Fourier transform infrared spectroscopy; ESI MS: Electrospray ionization mass spectrometry.
Table 3: Study findings on characterization of BVT1.
The crude extracts and fractions of methanol extract were evaluated for anxiolytic activity through EPM model only. After obtaining the impressive results to F3.2 with EPM model at 40 mg/kg b.w., further F3.2 was evaluated to know the optimal dosage in EPM model at dosages 40-100 mg/kg b.w. As the results in Table 4, the BVT1 had showed dose dependent and more activity at 80 mg/kg b.w. and is comparable with the standard drug diazepam with more entries and maximum time spent in open arms of the EPM i.e., 6.8±0.40 and 13.8±0.30 sec and 7.2±0.4 and 14.5±0.42 sec. After 80 mg dosage the activity was saturated i.e., at 100 mg/kg dose entries and time spent in open arms was observed is 5.6±0.21 and 10.3±0.21 less than the activity at 60 mg/kg b.w.
| Treatment | Dose (mg/kg) | Total open arms entries | Duration (sec) in open arms |
|---|---|---|---|
| Vehicle | 0.25 ml | 2.3±0.21b,# | 2.8±0.3b,# |
| Diazepam | 2 | 7.2±0.4a,# | 14.5±0.42a,# |
| BVT1 | 40 | 4.8±0.30a,b,# | 7.6±0.33a,b,# |
| BVT1 | 60 | 6.0±0.25a,# | 11.2±0.42a,b,# |
| BVT1 | 80 | 6.8±0.40a,# | 13.8±0.30a,# |
| BVT1 | 100 | 5.6±0.21a,#,b,@ | 10.3±0.21a,b,# |
Note: n=6, @: p<0.05, #: p<0.001, a: vs. control; b: vs. diazepam. Tukey's multiple comparison test is used after a one-way ANOVA
Table 4: Optimization of Anti-Anxiety of dose of BVT1.
The BVT1 was furthermore tested at dose 80 mg/kg b.w. for its anti-anxiolytic activity with other various models such as mirror chamber, light-dark and hole board tests. These models are widely used widely used behavior paradigm for screening anxiolytic agents in rodents[33-37]. The results of these test showed that the 80 mg/kg b.w. dosage of F3.2 possess anti-anxiolytic activity and is comparable with standard drug diazepam. BVT1 (80 mg/kg), the mirror chamber test clearly shows the anxiolytic activity of BVT1 in terms of reduced entry latency and extended duration in the mirror chamber (in comparison to the animals in the control group) during the test period as shown in Table 5.
| Treatment | Dose (mg/kg) | Latency (sec) to mirror chamber entry (Mean*±SEM) | Total entries in mirror chamber (Mean*±SEM) | Time spent (sec) in mirror chamber (Mean*±SEM) |
|---|---|---|---|---|
| Vehicle | 0.25 ml | 174.8±3.3b,# | 2.3±0.21b,# | 2.8±0.3b,# |
| Diazepam | 2 | 43.6±2.8a,# | 11.2±0.60a,# | 14.3±0.33a,# |
| BVT1 | 80 | 48.4 ± 2.4a,#,b,$ | 9.5±0.22a,#;b,@ | 13.2±0.61a,#;b,@ |
Note: n=6, @: p<0.05, $: p<0.01, #: p<0.001, a: vs. control; b: vs. diazepam. Tukey's multiple comparison test is used after a one-way ANOVA.
Table 5: Anti-Anxiety activity of BVT1 using mirror chamber test.
The light and dark model is based on mice's inherent aversion to brightly light unfamiliar and unpleasant environments[36]. The duration spent by the animal in the highly illuminated chamber is thought to be the most consistent behavior. BVT1 (80 mg/kg) treated group had showed more duration of time spent in the light chamber and increased immobility period in the light zone. The effects were comparable with the standard drug diazepam (2 mg/kg). The results were showed in Table 6. Measurement of exploratory activity may be done using a hole-board test. BVT1 (80 mg/kg) significantly enhanced the frequency of head dips compared to control group and equal to standard drug diazepam (Table 7).
| Treatment | Dose (mg/kg) | Latency (sec) to leave light zone (Mean*±SEM) | Time spent (sec) in light zone (Mean*±SEM) |
|---|---|---|---|
| Vehicle | 0.25 ml | 15.2±0.94b,# | 56.6±1.01b,# |
| Diazepam | 2 | 48.6±1.20a,# | 132.4±1.10a,# |
| BVT1 | 80 | 43.8±0.802a,b,# | 126.8±0.60a,#;b,$ |
Note: n=6, $: p<0.01, #: p<0.001, a: vs. control; b: vs. diazepam. Tukey's multiple comparison test is used after a one-way ANOVA.
Table 6: Anti-Anxiety activity of BVT1 using Light/Dark test.
| Treatment | Dose (mg/kg) | Number of head dips (Mean*± SEM) |
|---|---|---|
| Vehicle | 0.25 ml | 26.4±1.01b,# |
| Diazepam | 2 | 56.2±1.44a,# |
| BVT1 | 80 | 54.6±0.80a,# |
Note: n=6, #: p<0.001, a: vs. control; b: vs. diazepam, Tukey's multiple comparison test is used after a one-way ANOVA
Table 7: Anti-Anxiety activity of BVT1 using Hole Board test.
The results of the current study reveal the presence of different phytochemical constituents in different extracts of the T. indica flower extracts. Apart from our study, many researchers have been reporting presence of various bioactive molecules in medicinal plants[38-42], the extracts had showed good anti-anxiolytic activity at different doses and methanol extract showed more activity. EPM model, is universally using method to know the anxiety as it uses natural stimulus i.e., fear of a new, brightly open spaces, and fear of balancing on narrow spaces on raised platform. The extracts of T. indica flower, the methanol extract fractions and the isolated fraction (BVT1) from F3 had showed prominent activity in reduction of anxiety as they increased the time spent and number of entries on open arm than control groups. The BVT1 compound was identified as flavonoid and its anti-anxiety activity was tested using EPM, hole board, mirror chamber, light/dark tests. Hole board test is founded on a supposition that head-dipping of animals is inversely proportional to their anxiety state in the moderately aversive environment[43]. Therefore, the increased number of head dips into the holes on the board means waned anxiety state. Diazepam is also significantly increased the number of head-dips in the hole-board test as compared to control. The results of the light and dark and mirror chamber models also impressive and confirm the extracts and isolated fraction (BVT1) have significant anti-anxiolytic activity. There were other reports about anti-anxiolytic activity of different flavonoid compounds in different medicinal plants[43-46]. The anti-inflammatory and analgesic activities of T. indica flowers were reported by Komakech R et al.[47]. But as earlier said, there are very few reports on other biological activities of T. indica flowers.
The current study is a primary report in revealing the presence of flavonoids in T. indica flowers which is ought to show significant anxiolytic activity in various screening models. The activity was observed to be the same as that of standard anxiolytic drug diazepam (2 mg/kg p.o.). However, there is a need to do a thorough investigation in finding out the mechanism of action whether it is either through the serotonergic or GABAergic pathway. BVT1 has promising potential as an anti-anxiety drug, according to current research, which supports the traditional usage of T. indica flowers in different ailments.
Acknowledgements:
The authors would like to thank Dr. D. Senthil Kumar, Annamalai University for his excellent assistance and guidance. The authors are grateful to Guru Nanak Institute of Technical Campus-School of Pharmacy, Hyderabad for their support and assistance in providing the finest research facilities.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Kaur D, Shri R, Kamboj A. Evaluation of Anti-Anxiety Effect of Brassica oleracea L. Extracts in Experimental Animals. Pharmacogn J 2017;9(5):638-43.
- Mendonça Netto S, Warela RW, Fechine MF, Queiroga MN, Quintans-Júnior LJ. Anxiolytic-like effect of Rauvolfia Ligustrina Willd. Ex Roem. & Schult., Apocynaceae, in the elevated plus-maze and hole-board tests. BJP 2009;19(4):888-92.
- Yadav AV, Kawale LA, Nade VS. Effect of Morus alba L.(mulberry) leaves on anxiety in mice. Indian J Pharmacol 2008;40(1):32.
[Crossref] [Google Scholar] [PubMed]
- Munir S, Takov V, Coletti VA. Generalized anxiety disorder (nursing). StatPearls; 2022.
- Bandelow B, Michaelis S, Wedekind D. Treatment of anxiety disorders. Dialogues Clin Neurosci 2017;19(2):93-107.
[Crossref] [Google Scholar] [PubMed]
- Cassano GB, Rossi NB, Pini S. Psychopharmacology of anxiety disorders. Dialogues Clin Neurosci 2002;4(3):271-85.
[Crossref] [Google Scholar] [PubMed]
- Garakani A, Murrough JW, Freire RC, Thom RP, Larkin K, Buono FD, et al. Pharmacotherapy of anxiety disorders: Current and emerging treatment options. Front Psychiatry 2020;11:595584.
[Crossref] [Google Scholar] [PubMed]
- Baumel WT, Lu L, Huang X, Drysdale AT, Sweeny JA, Gong Q, et al. Neurocircuitry of treatment in anxiety disorders. Biomark Neuropsychiatry 2022;100052.
[Crossref] [Google Scholar] [PubMed]
- National Institute for Health and Care Excellence (Great Britain). Social anxiety disorder: recognition, assessment and treatment. National Institute for Health and Care Excellence (NICE); 2013.
- Balon R, Starcevic V. Role of benzodiazepines in anxiety disorders. Adv Exp Med Biol 2020;1191:367-88.
[Crossref] [Google Scholar] [PubMed]
- Ekor M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol 2014;4:177.
[Crossref] [Google Scholar] [PubMed]
- Talluri MR, Gummadi VP, Battu GR, Killari KN. Evaluation of hepatoprotective activity of Zanthoxylum armatum on Paracetamol-induced liver toxicity in rats. Indian j Pharm Sci 2019;81(1): 138-45.
[Crossref] [Google Scholar] [PubMed]
- Wachtel-Galor S, Benzie IF. Herbal Medicine: An Introduction to Its History, Usage, Regulation, Current Trends, and Research Needs. Biomolecul Clinic Aspects, 2011.
[Google Scholar] [PubMed]
- Demeke CA, Woldeyohanins AE, Kifle ZD. Herbal medicine use for the management of COVID-19: A review article. Metabol Open 2021;12:100141.
[Crossref] [Google Scholar] [PubMed]
- Ang L, Song E, Lee HW, Lee MS. Herbal medicine for the treatment of coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis of randomized controlled trials. J Clin Med 2020;9(5):1583.
[Crossref] [Google Scholar] [PubMed]
- Alzobaidi N, Quasimi H, Emad NA, Alhalmi A, Naqvi M. Bioactive compounds and traditional herbal medicine: Promising approaches for the treatment of dementia. Degener Neurol Neuromuscul Dis 2021;11:1-14.
[Crossref] [Google Scholar] [PubMed]
- Adeleye OA, Bamiro OA, Bakre LG, Odeleye FO, Adebowale MN, Okunye OL, et al. Medicinal plants with potential inhibitory bioactive compounds against coronaviruses. Adv Pharm Bull 2022;12(1):7-16.
[Crossref] [Google Scholar] [PubMed]
- Hossen MA, Ali Reza ASM, Amin MB, Nasrin MS, Khan TA, Rajib MH, et al. Bioactive metabolites of Blumea lacera attenuate anxiety and depression in rodents and computer-aided model. Food Sci Nutr 2021;9(7):3836-51.
[Crossref] [Google Scholar] [PubMed]
- Fonseca LR, Rodrigues RD, Ramos AD, da Cruz JD, Ferreira JL, Silva JR, et al. Herbal Medicinal products from Passiflora for anxiety: An unexploited potential. Sci World J 2020:6598434.
[Crossref] [Google Scholar] [PubMed]
- Kessler RC, Soukup J, Davis RB, Foster DF, Wilkey SA, van Rompay MI, et al. The use of complementary and alternative therapies to treat anxiety and depression in the United States. Am J Psychiatry 2001;158(2):289-94.
[Crossref] [Google Scholar] [PubMed]
- Moquin B, Blackman MR, Mitty E, Flores S. Complementary and alternative medicine (CAM). Geriatr Nurs 2009;30(3):196-203.
[Crossref] [Google Scholar] [PubMed]
- Tabuti JR. Herbal medicines used in the treatment of malaria in Budiope county, Uganda. J Ethnopharmacol 2008;116(1):33-42.
[Crossref] [Google Scholar] [PubMed]
- Khanzada SK, Shaikh W, Sofia S, Kazi TG, Usmanghani K, Kabir A, et al. Chemical constituents of Tamarindus indica. Medicinal plant in Sindh. Pak J Bot 2008;40:2553-9.
- Tatipamula VB, Killari KN, Gopaiah KV, Ketha A. GC-MS analysis of ethanol extract of Taxithelium napalense (Schwaerg) Broth along with its alpha-glucosidase inhibitory activity. Indian J Pharm Sci 2019;81:569-74.
- Talluri MR, Ketha A, Battu GR, Tadi RS, Tatipamula VB. Protective effect of Aurelia aurita against free radicals and streptozotocin-induced diabetes. Bangladesh J Pharmacol. 2018;13:287-295.
- Zu YG, Liu XL, Fu YJ, Wu N, Kong Y, Wink M. Chemical composition of the SFE-CO2 extracts from Cajanus cajan (L.) Huth and their antimicrobial activity in vitro and in vivo. Phytomedicine 2010;17(14):1095-101.
[Crossref] [Google Scholar] [PubMed]
- Kulkarni SK, Reddy DS. Animal behavioral models for testing antianxiety agents. Methods Find Exp Clin Pharmacol. 1996;18(3):219-30.
[Google Scholar] [PubMed]
- Kulkarni J. Book Review: Schizophrenia: Comprehensive treatment and management. Aust N Z J Psychiatry 2002;37(3):389.
- Costa-Campos L, Dassoler SC, Rigo AP, Iwu M, Elisabetsky E. Anxiolytic properties of the antipsychotic alkaloid alstonine. Pharmacol Biochem Behav 2004;77(3):481-9.
[Crossref] [Google Scholar] [PubMed]
- Luck SJ, Vogel EK. The capacity of visual working memory for features and conjunctions. Nature 1997;390(6659):279-81.
[Crossref] [Google Scholar] [PubMed]
- Reddy VV, Thalluri B, Rao Garige BS, Manda RM, Bakshi V. Isolation, characterization and assessment for central nervous system effects of novel phytomolecule from Galphimia glauca cav. stems. Asian J Chem 2019;31(6):1230-6.
- Balakrishna V, Senthilkumar D, Thakkalapally L. Evaluation of anxiolytic potential of various extracts of Tamarindus indica flowers. Asian J Pharm Clin Res 2020;13(11):136-38.
- Maiti P, Manna J, Dunbar GL. Current understanding of the molecular mechanisms in Parkinson’s disease: Targets for potential treatments. Transl Neurodegen 2017;6:28.
[Crossref] [Google Scholar] [PubMed]
- Brown GR, Nemes C. The exploratory behaviour of rats in the hole-board apparatus: Is head-dipping a valid measure of neophilia? Behav Processes 2008;78(3):442-8.
[Crossref] [Google Scholar] [PubMed]
- Wei XY, Yang JY, Wang JH, Wu CF. Anxiolytic effect of saponins from Panax quinquefolium in mice. J Ethnopharmacol 2007;111(3):613-8.
[Crossref] [Google Scholar] [PubMed]
- Peng WH, Hsieh MT, Lei YS, Liu YC, Liao J. Anxiolytic effect of seed of Ziziphus jujbuba in a mouse model of anxiety. J Ethnopharmacol 2010; 72:435-41.
[Crossref] [Google Scholar] [PubMed]
- Herrera-Ruiz M, Jiménez-Ferrer JE, De Lima TC, Avilés-Montes D, Pérez-García D, González-Cortazar M, et al. Anxiolytic and antidepressant-like activity of a standardized extract from Galphimia glauca. Phytomedicine 2006;13(1-2):23-8.
[Crossref] [Google Scholar] [PubMed]
- Singh J, Kumar A, Sharma A. Antianxiety activity guided isolation and characterization of bergenin from Caesalpinia digyna Rottler roots. J Ethnopharmacol 2017;195:182-187.
[Crossref] [Google Scholar] [PubMed]
- Halder S, Anand U, Nandy S, Oleksak P, Qusti S, Alshammari EM, et al. Herbal drugs and natural bioactive products as potential therapeutics: A review on pro-cognitives and brain boosters perspectives. Saudi Pharm J 2021;29(8):879-907.
[Crossref] [Google Scholar] [PubMed]
- Manganyi MC, Bezuidenhout CC, Regnier T, Ateba CN. A chewable cure "Kanna": Biological and pharmaceutical properties of Sceletium tortuosum. Molecules 2021;26(9):2557.
[Crossref] [Google Scholar] [PubMed]
- Jeyasri R, Muthuramalingam P, Suba V, Ramesh M, Chen JT. Bacopa monnieri and their bioactive compounds inferred multi-target treatment strategy for neurological diseases: A cheminformatics and system pharmacology approach. Biomolecules 2020;10(4):536.
[Crossref] [Google Scholar] [PubMed]
- Brown GR, Nemes C. The exploratory behaviour of rats in the hole-board apparatus: Is head-dipping a valid measure of neophilia? Behavioural Processes 2008;78(3):442-8.
[Crossref] [Google Scholar] [PubMed]
- Herrera-Ruiz M, Román-Ramos R, Zamilpa A, Tortoriello J, Jiménez-Ferrer JE. Flavonoids from Tilia americana with anxiolytic activity in plus-maze test. J Ethnopharmacol 2008;118(2):312-7.
[Crossref] [Google Scholar] [PubMed]
- Zhang LM, Yao JZ, Li Y, Li K, Chen HX, Zhang YZ, et al. Anxiolytic effects of flavonoids in animal models of posttraumatic stress disorder. Evid Based Complement Alternat Med 2012;2012:623753.
[Crossref] [Google Scholar] [PubMed]
- Pannu A, Sharma PC, Thakur VK, Goyal RK. Emerging role of flavonoids as the treatment of depression. Biomolecules. 2021 Dec 3;11(12):1825.
[Crossref] [Google Scholar] [PubMed]
- Liu Z, Silva J, Shao AS, Liang J, Wallner M, Shao XM, et al. Flavonoid compounds isolated from Tibetan herbs, binding to GABAA receptor with anxiolytic property. J Ethnopharmacol 2021;267:113630.
[Crossref] [Google Scholar] [PubMed]
- Komakech R, Kim YG, Matsabisa GM, Kang Y. Anti-inflammatory and analgesic potential of Tamarindus indica Linn.(Fabaceae): A narrative review. Integr Med Res 2019;8(3):181-6.
[Crossref] [Google Scholar] [PubMed]