- *Corresponding Author:
- Pracheta Janmeda
Department of Bioscience and Biotechnology, Banasthali Vidyapith, Tonk, Rajasthan 304022
E-mail: pracheta@banasthali.in
| Date of Received | 26 January 2021 |
| Date of Revision | 13 October 2022 |
| Date of Acceptance | 01 February 2023 |
| Indian J Pharm Sci 2023;85(1):93-106 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Oxidative stress related disorders occur as a result of free radicals in the cellular organs. Antioxidants provide protection contrary to oxidative stress by counteracting free radicals through the help of different bioactive constituents present in the plants. The aim of this study is to find out the quantitative estimation of phytochemicals (phenolics, tannins, flavonoid, non-tannins, alkaloid and saponins), in vitro antioxidant activity (2, 2-diphenyl-1-picryl-hydrazyl-hydrate, metal chelating, ferric reducing, superoxide dismutase and total antioxidant activity) of different parts of Cyperus rotundus. Along with this, the extraction of polyphenolic compounds by conventional and non-conventional methods and their optimization by response surface methodology technique were also studied. As the outcomes of the study, maximum amount of total phenolics content was found in the ethanolic fraction of stem (37.88±0.94 mg gallic acid equivalent/g). The root has shown the highest flavonoid (0.24±0.03 mg QAE/g) and non-tannin content (21.55±2.43 mg gallic acid equivalent/g) in the extract of petroleum ether. The total alkaloids and tannin content in flower and root i.e., 5.05±0.08 mg caffeine equivalent/g and 76.28±0.62 mg tannic acid equivalent/g was found to be better in aqueous extract respectively. The fraction of ethyl acetate has reported the greatest saponin content (39.2±3.8 mg gallic acid equivalent/g). In case of flower part half-maximal inhibitory concentration value of 2, 2-diphenyl-1-picryl-hydrazyl-hydrate and superoxide dismutase antioxidant activity varies from 0.57-1.90 mg/ml and from 0.053-1.401 mg/ml respectively. Antioxidant activity of each part of the plant was reported in the fraction of ethanol and water by fluorescence recovery after photobleaching (0.403±0.009-0.886±0.02 mg/ml), metal chelation activity (0.010±0.0-0.306±0.001 mg/ml) and total antioxidant capacity (0.030±0.0-0.317±0.002 mg/ml). Microwave assisted extraction method was found to be the suitable one for optimization of total phenolic content. So, the relationship between different parameter was determined through response surface methodology technique and evaluated by plotting the graphs. Polar extracts have found to show the maximum quantity of secondary bioactive metabolites and antioxidant activity as compared to non-polar one. And among the comparison between conventional and non-conventional methods, ultra sonication (nonconventional method) has shown the highest yield of bioactive phenolic compound. So, this study shows that Cyperus rotundus has wide range of secondary metabolites showing antioxidant properties that can be applied for different therapeutic applications.
Keywords
Antioxidant, Cyperus rotundus, in vitro, microwave-assisted, phytochemicals, resonance surface methodology
From ancient time, different parts of plant have been used against many ailments because of their curative effects. In India, around 95 % of the prescribed medicines are based on the conventional system of Siddha, Homeopathy, Ayurveda and Unani[1,2]. Herbal plants were utilized as a source for the various ailments of treatment in their own personalized way and that information keep on transferred from one generation to another[3]. Health concerns regarding the safety of synthetic agents draw the focus of investigators towards the use of natural phytochemicals which have higher commercial and pharmaceutical value[3,5]. These phytonutrients or the secondary metabolites provide various health benefits such as they may have anti-hypersensitive, cancer preventive, anti-diabetic, anti-inflammatory and antimicrobial properties[6]. Free radicals are produced along with reactive oxygen species via a causative agent known as oxidative stress. The oxidation in in vivo and in vitro that leads to nucleotide damage and caused several diseases like neural disorder, Alzheimer, cancer, cardiovascular diseases and liver diseases is initiated by free radicals[3,4]. Medicinal plants synthesized wide range of antioxidant molecules to contend the oxidative stress directed ailments[7,8]. Antioxidant molecules like reduced Glutathione, Superoxide Dismutase (SOD), catalase and many more can be synthesized inside the body or procured through the diet. The key source of these exogenous dietary antioxidants is medicinal plants. Either through supplementation of external antioxidant molecules or by boosting the defense of internal antioxidant enzyme, herbal plants show its very important role in human well-being [9].
Natural antioxidant or bio-active compounds can be extracted via different methods on the basis of effective process condition. There are many other types of extraction processes like maceration, ultrasonication, soxhletion and more are available, that will affect the phytochemical behavior of the plant[10]. Therefore, selection of an extraction method and conditions for maximum extraction of phytochemical is an important step.
Cyperus rotundus (C. rotundus) Linn is a common weed which is broadly distributed in tropical and sub-tropical area across global. It is known by the name of “Xiangfu” in the traditional system of China, “Musta moola churna” in the Ayurvedic system of India, as a “Nut grass” in the English and commonly known as ‘Motha’ in Hindi. Flavonoids, essential oil, alkaloid, starch, glycosides, furochromones and tannin are the main constituents responsible for C. rotundus therapeutic and biological properties. It is a multivalent weed reported for its various pharmacological properties such as anti-inflammatory, anti-pyretic, anti-diabetic, tranquilizing effect, antibacterial, analgesic and more[11,12].
This study put forward a wide range of screening and estimations of secondary bioactive metabolites in different plant parts under study. Comparative study of conventional and non-conventional extraction methods has been evaluated. Finally, Resonance Surface Methodology (RSM) is used to standardize the terms for the maximum secondary metabolite extraction.
Materials and Methods
The chemical, reagents and solvents consumed in this study were purchased from HI Media, Mumbai and Merek Co. (Germany), Mumbai. The other chemicals and reagents without label were of analytic grade and are used without further purification. The instruments used are Digital balance (Blue Star HF 271, Denmark and REMI College 13154), Hot air oven (Mvtex Universal (220v/50HZ), Rotatory evaporator (Heidolph-G3, GERMANY), Spectrophotometer (EC Ultraviolet- Visible (UV-Vis) Double Beam spectrophotometer 5704SS and Systronics-5816), Weighing machine (CE Model-125), Centrifuge (College B154 Mettler- Toledo), Microwave oven (Magiccook20S, whirlpool microwave 850 W), and UV-Sonicator (PCi Analysis).
Experimental plant and extract preparation:
The stem, root and flower of C. rotundus were collected in the month of October from the botanical gardening area of Banasthali Vidyapith, Rajasthan, India. The collected plant was identified morphologically with help of already existing literature and validated by Krishi Vigyan Kendra’s botanist of Banasthali Vidyapith, Rajasthan (validation no.BURI-2019-41).
The different extracts with various solvents i.e., petroleum ether, benzene, chloroform, ethyl acetate, ethanol and water were prepared using Soxhlet apparatus of different parts of C. rotundus plant. Extracts are dried and kept in desiccators and then stored in air tight containers at 4° to quantify the secondary metabolites[13]. The 1 mg/ml concentration of extract was used for all the experimental analysis.
Quantitative phytochemical assays:
The quantitative contents of different phytochemicals were estimated with various methods. The Total Phenolic Content (TPC) was estimated using the Folin Ciocalteu method with some modification[14,15]. Gallic Acid Equivalent (GAE) per 50 g of fresh mass was calculated in finding plant’s TPC with the help of formula:
C=c.V/m (1)
Where C=Total content of plant extract in mg/g, c=Concentration established from standard curve (y), V=Volume of extract (ml), m=Weight of pure extract (g)
The Total Flavonoid Content (TFC) was determined by method of Olajire et al.[16]. Standard curve of quercetin was prepared and the content was calculated using above equation and quantified as Quercetin Equivalents (QE) (mg quercetin/g dried extract). The Total Tannins Content (TTC) was quantified by the Folin’s-Denis method of Sadasivam et al.[17] with slight modification. The results were expressed as percentage of Tannic Acid Equivalent (TAE) per 50 g of fresh biomass using above equation. The Total Non-Tannin Content (TNC) determined was done by using polyvinylpolypirrolidone, an insoluble compound that fix[18,19]. With the help of gallic acid’s standard curve, total content of non-tannin compounds in plant extracts in GAE per 50 g of fresh mass was estimated. The Total Alkaloid Content (TAkC) was determined according to Manjunath’s UV Spectrophotometer method[20]. Total content of alkaloid compound was expressed as Caffeine Equivalent (CE)/50 g of fresh mass. The Total Saponin Content (TSC) in plant parts was estimated by modified method as explained by Jain et al.[21,22]. Then the total saponin compounds content in plant extracts was calculated as Saponin Quillaja Equivalent (SQE) per 50 g of fresh mass using above equation[23].
Evaluation of antioxidant properties:
Free radical 2,2-Diphenyl-1-Picryl-Hydrazyl- Hydrate (DPPH) scavenging assay was determined by Blois method[24,25]. The DPPH scavenging reaction mechanism is written as: (DPPH•)+(H-A)=DPPHH+( A•). Percentage (%) of inhibition can be computed by: Acontrol-Atest/ Acontrol×100
Where Atest=Absorbance of sample, Acontrol=Absorbance of control. By using the linear regression analysis, concentration of extract was obtained along with 50 % Half Maximal Inhibitory Concentration (IC50).
SOD assay was used as described by Nishikimi et al.[26-28] and it’s IC50 value was determined from the graph by plotting the inhibition percentage vs. the extract concentrations[29]. Metal chelating activity is estimated by the method of Dinis et al.[30] thru some modifications and was calculated in terms of mg/g standard equivalent with formula using equation 1. Ferric Reducing Antioxidant Potential (FRAP) was performed by using the method of Benzie and Strain, C. rotundus extracts were measured for their FRAP[31] and its activity was calculated with above equation in terms of mg/g equivalent of standard. The Total Antioxidant Capacity (TAC) was estimated by Prieto’s method with Phosphomolybdenum assay[32] and was calculated in terms of mg/g equivalent of standard.
Comparison of conventional method and nonconventional method:
Conventional extracting method is one of the oldest methods which usually performed by the use of different techniques like simple distillation, reflux, cold maceration and soxhlation[33]. The non-conventional extraction methods (ultrasound assisted and microwave assisted extraction) can produce higher product in short span of time by utilizing a smaller amount of solvent[34]. The optimization of innumerable biotechnological processes and determination of effects of numerous parameters can be done using, one of the most widely employed statistical and mathematical techniques RSM[35-39]. Different techniques were compared to extract bioactive compounds from stem of C. roduntus through the use of variable solvents (i.e., water, ethanol, chloroform, and petroleum ether) by the help of extraction method as described by Hijazi et al.[40] with some modifications. As per phytochemical quantitative assays, the aqueous fraction of stem has shown the highest quantity of TPC therefore, the stem powder has been used for the further experimentation.
Maceration method: 1 g of powdered stem of C. roduntus was blended with 50 ml of different solvents for variable time periods (6, 24 and 48 h) at room temperature with proper agitation. After the desired time period, and using 0.45 millipore of filter paper the extracts were filtered. Then, using a rotary evaporator under reduced pressure the extracts were thickened at 40°. Lastly, the extracts were stored at -20° after weighing till further use.
Reflux method: In every different round bottom flask, 50 ml of solvent was added with 1 g of dried sample each. For preparing extracts at different time period (6, 24 and 68 h), the mixture was stirred carefully and continuously for a given period of hours. Each extraction was repeated and performed carefully for this method with all the solvents under the dark condition, up to three times. Then, under the reduced pressure the extracts were filtered by Buchner funnel and have been taken in use for further experimentation.
Ultrasound Assisted Extraction (UAE): 1 g of powdered stem of C. roduntus was added in 100 ml of flask along with 50 ml solvents. Then for the extraction, UAE was performed at 0° at 100 W for 10, 25 and 60 min.
Microwave Assisted Method (MAE): In flat bottom threaded round top, Perfluoroalkoxy Alkanes (PFA) vials the plant samples (0.5 g) and solvents (25 ml) were mixed and kept into a PFA beaker in microwave oven. After some modifications in Pan’s method, the mixtures were treated at 650 W power from microwaves to achieve 2, 4 and 6 min of treatment by 30 s power on and off for first treatment and then by 15 s power on and off for rest of the time and repeated the above step[41]. Then after that, samples were cooled down at room temperature, and filtered quickly through a 0.45 μm membrane filter. Then, dried further by using a rotary evaporator at 40° under reduced pressure before measuring the dry weight of extract[42]. The percentage yield was calculated using following formula:
Percentage Extraction yield (w/w)=Weight of dried extract/Weight of the sample used for extraction×100
Optimization of total phenolic extraction:
The optimization of TPC was done on basis of RSM using central composite design. The parameters used were as follows; incubation time (2-6 min), pH (6-8) and substrate concentration (2 %-6 %, w/v). To fit the following polynomial second-order equation, response surface regression technique was used for the analysis of tested data[10].
Y=βk0+Σi=15βkixi+Σi=15βkiixi2+Σi=14Σj=i+15βkijxixj
Where, Y was TPC (mg GAE/g); xi and xj were the coded independent variables, the response influencer of Y variables and βk0, βki, βkii and βkij were constant coefficients[10].
Statistical analysis:
Samples and standards were analyzed in triplicates and results were expressed as mean±standard deviation. For the evaluation of Analysis of Variance (ANOVA), statistical analysis was performed. Minitab 15.0 (Minitab Inc., Pennsylvania, United States of America) was used for the RSM and regression analysis. The 3-Dimensional (3D) response surface plot and regression analysis of independent variable and each dependent variable were used to estimate optimal extraction conditions.
Results and Discussion
The extracts of different parts of plant have been prepared and used in the experiment. Its extractive value of all fractions was already reported by Prakash et al.[43] and in the ethanolic extracts for flower (6.63 %), roots (5.91 %) and stems (4.7 %) parts it is found to be in higher amount. Also, in a study on spearmint by Bimakr suggested that higher extraction yield is evaluated in polar solvent than in non-polar solvent[44]. Research on Trianthema portulacastrum L. also observed the maximum extractive value in aqueous extract and minimum in fraction of petroleum ether extract[45]. Therefore, among all plant parts, the ethanolic and aqueous extracts were used further for quantitative optimization studies.
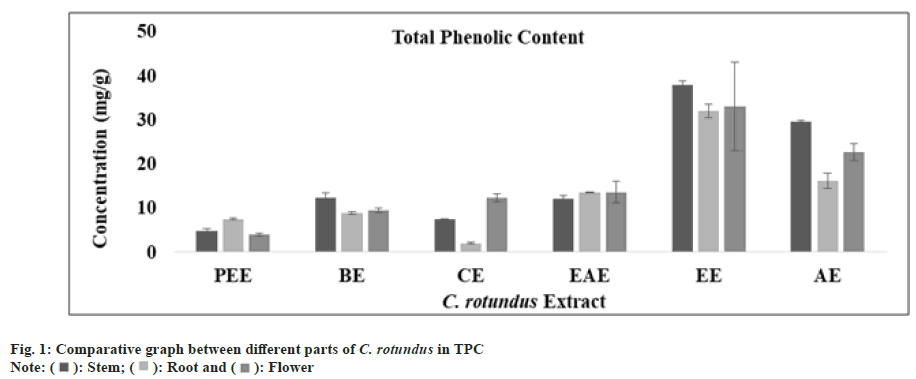
Phenolic compounds are abundant secondary bioactive metabolites available in plants. They are acknowledged to have a varied range of chemical and biological activities and it is likely that the activity of extracts was because of phenolic compound[46]. In all extract of stem, root and flower using regression equation of standard curve (y=0.0061x, R²=0.8387), the TPC (mg/g) was determined. All these amounts were expressed in GAE in Table 1. The TPC (mg/g) was found to be in appreciable amount in ethanolic extract of stem, root and flower but the greatest amount was analyzed in the ethanolic fraction of stem as predicted (fig. 1). High phenolic content has capability to raise the antioxidant activity of extract. Cocos nucifera kernel’s ethanolic and aqueous extracts were also composed of phenolic compounds and possessed greater antioxidant activity[47]. According to a research, daily ingestion of polyphenolic compound up to 1 g from fruits and vegetables rich diet resulted an inhibitory effect on the carcinogenesis and mutagenesis effect of tumor in human beings[48].
| Secondary metabolites | Sample | |||
|---|---|---|---|---|
| Stem | Root | Flower | ||
| TPC (mg GAE/g) | PetroleumEther extract | 04.71±0.51 | 07.46±0.15 | 03.94±0.25 |
| Benzeneextract | 12.22±1.21 | 08.83±0.33 | 09.50±0.50 | |
| Chloroformextract | 07.32±0.17 | 01.99±0.16 | 12.26±0.87 | |
| EthylAcetate extract | 12.05±0.67 | 13.46±0.15 | 13.55±2.42 | |
| Ethanolextract | 37.88±0.94 | 31.93±1.50 | 32.93±10.07 | |
| Aqueousextract | 29.60±0.25 | 16.16±1.69 | 22.60±1.91 | |
| TFC (mg QAE/g) | PetroleumEther extract | 0.040±0.01 | 0.243±0.03 | 0.002±0.01 |
| Benzeneextract | 0.019±0.01 | 0.024±0.01 | 0.008±0.01 | |
| Chloroformextract | 0.009±0.01 | 0.030±0.00 | 0.029±0.03 | |
| EthylAcetate extract | 0.028±0.01 | 0.075±0.02 | 0.196±0.02 | |
| Ethanolextract | 0.016±0.01 | 0.003±0.01 | 0.021±0.01 | |
| Aqueousextract | 0.061±0.02 | 0.206±0.05 | 00.13±0.02 | |
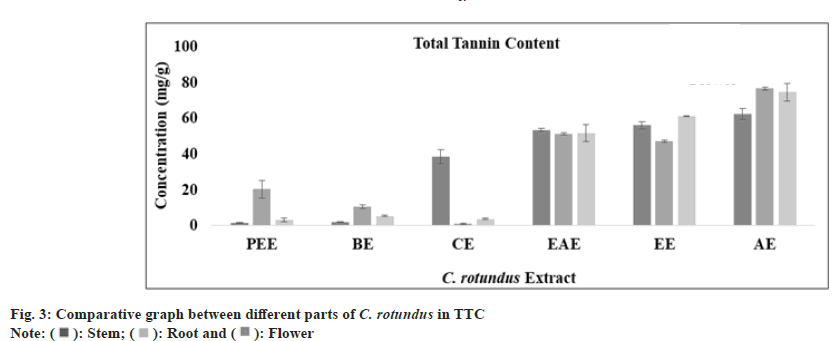
| TTC (mg TAE/g) | Petroleumether extract | 01.47±0.21 | 20.04±4.92 | 02.90±1.08 |
| Benzeneextract | 01.76±0.21 | 10.13±1.03 | 05.04±0.46 | |
| Chloroformextract | 38.23±4.04 | 0.803±0.43 | 03.47±0.50 | |
| Ethylacetate extract | 53.04±0.95 | 50.85±0.62 | 51.32±4.83 | |
| Ethanolextract | 55.75±2.08 | 46.94±0.50 | 60.85±0.38 | |
| Aqueousextract | 62.04±3.22 | 76.28±0.62 | 74.37±4.97 | |
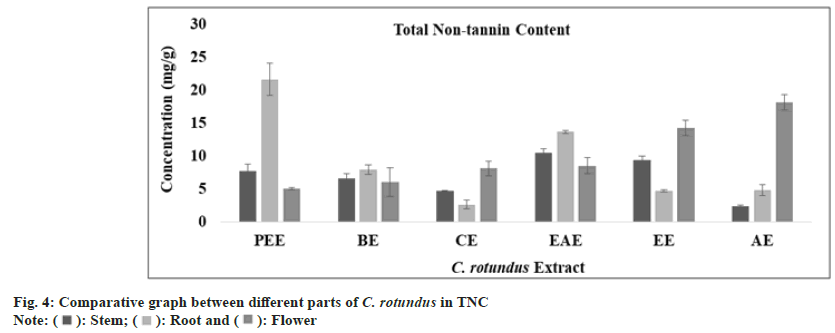
| Total non-tannin content (mg GAE/g) | Petroleumether extract | 07.61±1.05 | 21.55 ± 2.43 | 04.99±0.16 |
| Benzeneextract | 06.54±0.73 | 07.88±0.70 | 05.97±2.17 | |
| Chloroformextract | 04.66±0.11 | 02.60±0.69 | 08.05±1.10 | |
| Ethylacetate extract | 10.48 ± 0.61 | 13.60 ± 0.25 | 08.48 ± 1.18 | |
| Ethanolextract | 09.30±0.62 | 04.65±0.15 | 14.16±1.16 | |
| Aqueousextract | 02.32±0.17 | 04.77±0.85 | 18.05±1.16 | |
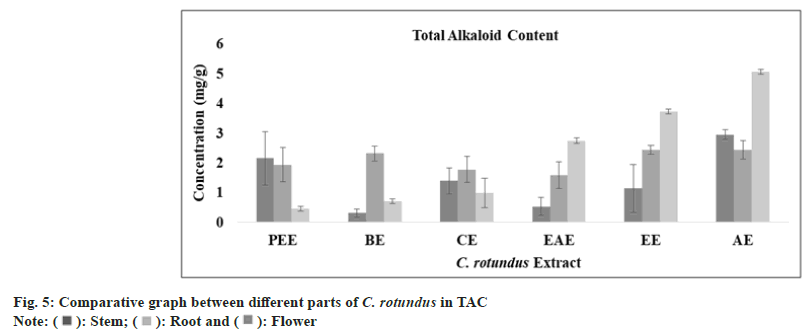
| TAkC (mg CE/g) | Petroleumether extract | 02.15±0.90 | 01.93±0.58 | 00.45±0.08 |
| Benzeneextract | 00.31 ± 0.14 | 02.31 ± 0.25 | 00.70±0.08 | |
| Chloroformextract | 01.39±0.44 | 01.77±0.44 | 00.99±0.50 | |
| Ethylacetate extract | 00.53±0.30 | 01.58±0.45 | 02.74±0.10 | |
| Ethanolextract | 01.13±0.80 | 02.43±0.15 | 03.73±0.08 | |
| Aqueousextract | 02.95±0.16 | 02.44±0.31 | 05.05±0.08 | |
| TSC (mg SQE/g) | Petroleumether extract | 08.93 ± 0.23 | 09.06±4.43 | 19.46±0.30 |
| Benzeneextract | 06.26±4.27 | 16.00±4.27 | 20.20±0.80 | |
| Chloroformextract | 10.73±1.10 | 07.60±1.40 | 23.53±3.10 | |
| Ethylacetate extract | 11.80±0.20 | 21.13±3.90 | 31.26±1.20 | |
| Ethanolextract | 24.00±9.67 | 32.07±7.73 | 18.86±2.53 | |
| Aqueousextract | 17.86±1.72 | 39.20±3.80 | 21.06±4.05 | |
Table 1: In Vitro Quantitative Phyto-Chemical Assays
Flavonoids and flavonols are also very important natural compounds of plants which work as potent metal chelators and free radical scavengers[49]. They are one amongst diverse collections of natural phenolics[47,49,50]. Flavonoids are low molecular weight substances and are usually subdivided into flavonols, flavones, anthocyanidins and chalcones. The alkoxylation and hydroxylation pattern of the A-B rings of these compounds vary broadly and are of great importance in determining their activity as antioxidants. In general, plant antioxidant properties rely on ability of donation of electron or hydrogen to a free radical[50]. The TFC (mg/g) in all extract of stem, root and flower was determined with regression equation of standard curve (y=0.5808x, R²=0.9601). All these amounts were expressed in QE and TFC was calculated with standard curve in Table 1. The appreciable amount of TFC was found in aqueous extract of stem, petroleum ether extract of root and ethyl acetate extract of flower part of C. rotundus as shown in fig. 2.
Tannins are polyphenolic compound which are known to prevent the adherence of bacteria to the walls of urinary tract in the urinary tract infection. Combination of anthocyanins with tannin can breakdown atherosclerotic plaques and oxidized cholesterol in the blood stream. Tannins have also reported to have broad range of properties like astringent, healing of inflamed mucous membrane and woundhasten property. Muthukumaran et al.[51] presented the highest content of tannin in the ethanolic fraction of Peltophorum pterocarpum. In Cedrus deodara, the maximum amount of tannin content was reported in aqueous fraction of heart wood. The total tannin content (mg/g) in all extract of stem, root and flower was determined from regression equation of standard curve (y=0.0073x, R²=0.9081). All these amounts were expressed in TAE in Table 1. The TTC was found to be highest in aqueous extract of stem, root and flower of plant C. rotundus (fig. 3). Ethyl acetate extract of stem, petroleum ether extract of root and aqueous extract of flower has exhibited an optimum amount of non-tannin content as predicted by fig. 4 and the standard graph of GAE was used for calculations.
Alkaloids are a diverse group of secondary metabolites with great antimicrobial activity Bonjean et al.[52]. It is reported that alkaloids provide protection against chronic diseases and are also capable in reducing headaches associated with hypertension[53]. A study revealed that alkaloid from C. rotundus has been extracted and found to have protective role against gastro-intestinal tumor[54]. The optimum amount of alkaloid has been calculated with CE (y=0.0191x, R²=0.9815) and the highest content was observed in aqueous extract of stem (2.95±0.16 mg/ml), root (2.44±0.31 mg/ml) and flower (5.05±0.08 mg/ml), as shown in fig. 5 and Table 1.
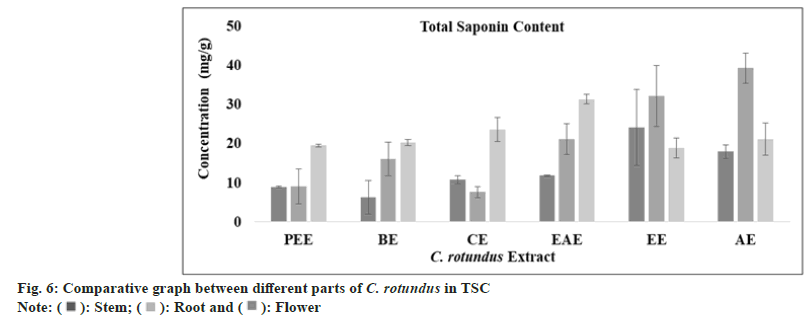
The saponins metabolites are categorized into glycoside group of compounds which comprised of aglycon (triterpene-steroid) and glycon (hexose-uronic acid). They are characterized by their hemolytic activity and foaming properties. From 100 families of plants, around 150 types of saponins were reported to have anti-cancer activity[55]. The amount of saponin (TSC) has been calculated by (y=0.005x, R2=0.8931) using linear regression curve as standard graph and was found highest in aqueous extract of stem and root (17.86±1.72 mg/ml; 39.2±3.8 mg/ml) and ethyl acetate extract of flower (31.26±1.2 mg/ml) as the value are tabulated in Table 1 and presented in fig. 6.
Antioxidant assays are most widely used method to evaluate the drug potential of a medicinal plant applied in degenerative diseases. The plant extract used for the experiment were aqueous and ethanolic extract of stem, root and flower. As all of these extracts manifesting wide range of secondary metabolites, DPPH scavenging assay was used to determine the antioxidant ability of C. rotundus various extracts. Anggraini et al.[56] studied in his research about property properties of Lepisanthes alata and found to have the color enhancing and antioxidant value and can be used as food additive. All the extracts were efficient in reducing purple colored DPPH to yellow colored DPPH, which was measured as percentage inhibition and IC50 (fig. 7 and Table 2). IC50 has been widely used to determine the efficacy of variable drugs. It indicates the amount of drug is required to inhibit a biological process by half[57]. Higher anti-oxidant activity is indicated by lower IC50 value. Hence, the scavenging effect of different extracts and Butylated Hydroxytoluene (BHT) standard (1 mg/ ml) concentration was found to be in following order AEF>AES>EEF>EES>EER>AER. Aqueous extracts of stem and flower has shown the strongest DPPH scavenging activity than BHT standard while the other represented the moderate antioxidant activity. According to present study, the concentration of extract and percentage inhibition showed a linear relationship which means that the higher the concentration of plant extract the more is the scavenging property which is similar to the Bui et al. [58].
| Concentration(mg/ml) | IC50 | ||||||
|---|---|---|---|---|---|---|---|
| Standard | EES | AES | EEF | AEF | EER | AER | |
| DPPH | 0.065 | 0.830 | 0.630 | 0.75 | 0.57 | 1.23 | 1.90 |
| SOD | 0.144 | 0.726 | 0.361 | 0.053 | 0.620 | 1.401 | 1.293 |
Table 2: Free Radical Scavenging Activity (% Inhibition) of Standard and Different Extracts of C. rotundus
Superoxide radicals are the main source of reactive oxygen species. They generated the harmful singlet oxygen and hydroxyl radicals which give rise to oxidative stress[59]. The scavenging of superoxide anions effects the flavonoid’s antioxidant properties[60]. Lim et al.[61] determined that the ethanolic fraction of Sargassum serratifolium possessed strong superoxide radical scavenging activity as compared to the other extracting fractions and can be used as natural antioxidants source. The IC50 of extracts were shown in the following order: EEF>AES>AEF>EES>AER>EER. These indicated that the ethanolic extract of flower was reported to have lowest IC50 and therefore has shown the highest quenching activity. EEF was found to possessed effectively good scavenging activity for superoxide anions at all the respective concentrations. On the basis of comparative study of different extracts from the different parts of the C. rotundus that is tabulated in Table 2 and represented in fig. 8. It was determined that the aqueous stem extract and ethanolic flower extract exhibited comparatively good antioxidant property as compared to others.
The chelation of Ferrozine ion complex was studied at 562 nm for the estimation of metal chelating effect of C. rotundus extracts[30]. The ascorbic acid’s standard curve equation was y= 787x+0.208 and R2 value was found to be 0.961. By the help of standard equation, the metal chelation activity in terms of mg ascorbic acid equivalent/g was calculated for different extracts of C. rotundus with concentration dependent relationship. The maximum chelation was found in ethanol extract of stem (0.306±0.001) as mentioned in Table 3 and the decreasing order of metal chelating activity was determined to be EES>AES>AER>AEF>EER>EEF.
| Antioxidant activity | EES (1 mg/ml) | AES (1 mg/ml) | EEF (1 mg/ml) | AEF (1 mg/ml) | EER (1 mg/ml) | AER (1 mg/ml) |
|---|---|---|---|---|---|---|
| MCA | 0.306±0.001 | 0.133±0.000 | 0.01±0.003 | 0.066±0.001 | 0.037±0.002 | 0.132±0.002 |
| FRAP | 0.695±0.007 | 0.766±0.012 | 0.886±0.016 | 0.834±0.018 | 0.827±0.020 | 0.403±0.009 |
| TAC | 0.317±0.002 | 0.127±0.005 | 0.209±0.000 | 0.150±0.001 | 0.310±0.006 | 0.030±0.002 |
Table 3: Antioxidant Activities of Different Extract of Various Parts of C. rotundus
The capacity of C. rotundus extracts to reduce the ferric ion was determine by FRAP assay[31]. The reduction of ferric ion and TPTZ (FeIII-TPTZ) at low pH to ferrous ion (FeII) at 593 nm was found maximum in ethanol extract of flower (0.886±0.016 mg/ml). An increasing trend of ferric reduction activity was revealed in the study which corresponded to work of other researchers[61,62]. The standard curve equation from BHT was found to be, y=1.163x+1.226, with an R2 value of 0.931. FRAP activity in decreasing order was found to be EEF>AEF>EER> AES>EES>AER as mentioned in Table 3. All amounts were expressed as mg BHT equivalent/g of C. rotundus extracts of all the parts of plants.
The reduction of Mo (VI) to Mo (V) takes place due to antioxidant compound with the formation of a green color phosphate/Mo (V) complex that gave maximum absorbance at 695 nm[32]. TAC of stem, flower and root extracts of C. rotundus was obtained by regression equation from standard curve of Gallic acid, i.e. y=1.850x+0.399, with an R2 value of 0.925 (fig. 9). The study revealed an increasing trend of absorbance with increasing concentration, suggesting dose dependent relationship in the TAC of plant extracts. In ethanolic extract of stem, maximum TAC was found (0.317±0.002 mg/ml) as mentioned in Table 3. The decreasing order of TAC was found to be EES>EER>EEF>AEF>AES>AER. The capacity of total antioxidant of C. rotundus extracts were expressed as mg GAE/g.
The different antioxidant assays revealed that the stem extracts have better free radical scavenging, metal chelating and metal reducing effect. All these properties not only suggested the anti-carcinogenic but also other related and non-related pharmacological activities. Flower and root extracts also exhibited remarkable anti-oxidant properties but they are not so good in comparison to stem. It was observed in the recent study, that ethanol extract represented better antioxidant character than the aqueous extracts, signifying the relevancy of ethanol-based drugs[62-66]. Nutraceuticals that reduces the oxidative stress with antioxidant-rich plant extracts helps in prevention of degenerative diseases[67].
The percentage yield obtained and TPC calculated by maceration, reflux, ultra-sonication and MAE are tabulated in Table 4 and Table 5. The ultra-sonication method has showed the maximum yield after 6 min, 24 h in the aqueous extract of stem. Other methods (like reflux, maceration and microwave assisted) also have reported the maximum higher yield in the aqueous extract of stem but after the time period of 24 h. In stem, TPC was found higher in the aqueous and ethanolic extract at a time period of 6 h, 68 h, 48 h and 6 min in maceration, reflux, ultra sonication and microwaveassisted method respectively.
| Methods | Incubation time | Solvents | |||
|---|---|---|---|---|---|
| Pet ether | Chloroform | Ethanol | Water | ||
| Maceration | 6 h | 01.32±0.052 | 03.12±0.55 | 05.62±0.09 | 20.85±0.04 |
| 24 h | 01.70±0.01 | 05.29±0.03 | 09.48±0.03 | 18.10±0.05 | |
| 48 h | 01.40±0.01 | 00.021±0.02 | 07.00±0.08 | 19.30±0.02 | |
| Reflex | 6 h | 00.07±0.01 | 00.9±0.02 | 05.49±0.01 | 09.00±0.08 |
| 24 h | 01.49±0.01 | 04.10±0.01 | 06.70±0.02 | 14.80±0.14 | |
| 68 h | 0.30±0.01 | 01.97±0.05 | 07.00±0.08 | 14.02±0.04 | |
| Ultrasonication | 10 min | 10.40±0.01 | 03.79±0.01 | 05.59±0.03 | 18.00±0.02 |
| 25 min | 03.81±0.03 | 11.30±0.02 | 04.04±0.04 | 22.09±0.02 | |
| 60 min | 02.28±0.03 | 04.20±0.08 | 00.80±0.02 | 16.70±0.02 | |
| Microwaveassisted | 2 min | 02.23±0.01 | 01.55±0.01 | 04.50±0.01 | 16.71±0.21 |
| 4 min | 01.36±0.09 | 02.30±0.01 | 08.70±0.02 | 18.74±0.02 | |
| 6 min | 01.47±0.01 | 02.00±0.08 | 04.44±0.01 | 17.00±0.08 | |
Table 4: Summary of Total Yield Obtained by using Conventional and Non-Conventional Methods
| Methods | Incubation time | Solvent | |||
|---|---|---|---|---|---|
| Pet ether | Chloroform | Ethanol | Water | ||
| Maceration | 6 h | 03.33±0.13 | 02.44±0.28 | 09.14±0.41 | 27.16±0.27 |
| 24 h | 06.27±0.20 | 02.22±0.21 | 12.33±0.13 | 14.27±0.47 | |
| 48 h | 02.04±0.21 | 04.96±0.12 | 10.36±0.18 | 18.16±0.41 | |
| Reflex | 6 h | 08.55±0.27 | 03.77±0.14 | 12.33±0.15 | 13.40±0.14 |
| 24 h | 01.88±0.27 | 00.55±0.14 | 03.00±0.07 | 10.80±0.27 | |
| 68 h | 06.72±0.20 | 05.72±0.27 | 08.44±0.14 | 11.22±0.20 | |
| Ultrasonication | 6 h | 08.33±0.13 | 12.44±0.48 | 16.16±0.27 | 10.88±0.28 |
| 24 h | 09.00±0.13 | 14.61±0.21 | 11.88±0.21 | 08.89±0.28 | |
| 48 h | 07.50±0.13 | 07.22±0.21 | 21.77±0.34 | 07.17±0.27 | |
| Microwave assisted | 2 min | 98.72±0.48 | 14.00±0.49 | 32.38±0.28 | 111.80±0.98 |
| 4 min | 18.94±0.48 | 15.72±0.34 | 102.20±0.41 | 36.27±0.70 | |
| 6 min | 22.61±0.21 | 12.44±0.44 | 93.27±0.21 | 118.80±0.41 | |
Table 5: Optimization of Total Phenolic Compound using Conventional and Non-Conventional Methods
On the basis of above results, it was observed that MAE method is the suitable one for determining the yield percentage and TPC content. It was more effective than the heat reflux and extraction methods as a sample treatment technique[68]. Within few minutes while using conventional technique as compared to non-conventional extraction techniques, a large yield of extracted product was obtained. There are many advantages of MAE over other methods because of its higher extraction selectivity, less labor, higher extraction efficiency and lower extraction time. Hence, these characteristics make it a constructive way for the extraction of wide range of bioactive compounds from C. rotundus stems[40].
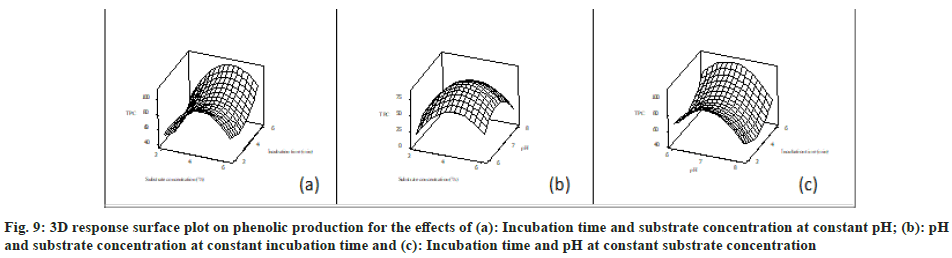
The polynomial quadratic equation coefficients were computed by using the experimental data along with all the experimental condition and responses obtained from Table 6. The polynomial equation of GAE has been used to calculate the TPC content after MAE. The experimental values and predicted value of TPC mg (GAE)/g were very similar. ANOVA is showed in Table 7 that illustrated the quadratic polynomial resultant models satisfactorily obtained the investigational data with multiple determination coefficient’s (R2)=0.7957, which signify that this regression was statistically significant (p<0.0001). There is insignificant lack of fit in the model, statistically (p=0.927). p-values commonly used for the analysis of all the regression coefficient’s significance of polynomial equation. The p-value and corresponding coefficient significance are inversely proportional to each other, i.e., lesser the p-values there are major significance of the corresponding coefficient. The estimated coefficient and corresponded p-values recommended that variation of pH and incubation time (microwave treatment time) had meaningful effect on the extraction of phenolics.
| Runorder | Substrate concentration(%, w/v) | pH | Incubation time (min) | TPC mg (GAE)/g | |
|---|---|---|---|---|---|
| Experimental | Predicted | ||||
| 1 | 2 | 6 | 2 | 28.500 | 29.542 |
| 2 | 6 | 6 | 2 | 38.667 | 40.075 |
| 3 | 2 | 8 | 2 | 23.833 | 19.508 |
| 4 | 6 | 8 | 2 | 31.167 | 33.042 |
| 5 | 2 | 6 | 6 | 45.5 | 43.625 |
| 6 | 6 | 6 | 6 | 65.833 | 70.158 |
| 7 | 2 | 8 | 6 | 24.000 | 22.592 |
| 8 | 6 | 8 | 6 | 53.167 | 52.125 |
| 9 | 2 | 7 | 4 | 33.167 | 39.733 |
| 10 | 6 | 7 | 4 | 66.333 | 59.767 |
| 11 | 4 | 6 | 4 | 68.000 | 63.100 |
| 12 | 4 | 8 | 4 | 44.167 | 49.067 |
| 13 | 4 | 7 | 4 | 67.667 | 83.813 |
| 14 | 4 | 7 | 4 | 70.833 | 83.813 |
| 15 | 4 | 7 | 4 | 55.333 | 83.813 |
| 16 | 4 | 7 | 4 | 69.667 | 83.813 |
| 17 | 4 | 7 | 4 | 95.833 | 83.813 |
| 18 | 4 | 7 | 4 | 95.333 | 83.813 |
| 19 | 4 | 7 | 4 | 108.000 | 83.813 |
| 20 | 4 | 7 | 4 | 107.833 | 83.813 |
Table 6: Experimental Design and Responses for Phenolics Extraction from C. rotundus
| Source | DFa | SeqSSb | AdjSSb | Adj MSc | F | p |
|---|---|---|---|---|---|---|
| Regression | 9 | 10597.4 | 10597.4 | 1177.49 | 3.84 | 0.024 |
| Linear | 3 | 2045.7 | 2617.4 | 872.45 | 2.85 | 0.091 |
| Square | 3 | 8358.7 | 8358.7 | 2786.25 | 9.09 | 0.003 |
| Interaction | 3 | 193.0 | 193.0 | 64.33 | 0.21 | 0.887 |
| Residual Error | 10 | 3064.4 | 3064.4 | 306.44 | - | - |
| Lack-of-Fit | 3 | 184.8 | 184.8 | 61.61 | 0.15 | 0.927 |
| Pure Error | 7 | 2879.6 | 2879.6 | 411.37 | - | - |
| Total | 19 | 13661.8 | - | - | - | - |
| R2 | 0.7957 % | 0.7738 % | - | - | - | - |
Table 7: Anova of RSM Model for TPC from C. rotundus stem
The mathematical expression of relationship of TPC (mg GAE/g) with distinctive variables (incubation time (i.t), pH variation and substrate concentration (s.c)) is as below in uncoded factor stems: TPC (mg GAE/g)=- 1343.42+66.51×s.c+385.19×pH-23.85i.t-8.52×s.c×s.c- 27.73×pH×pH+4.2×i.t×i.t+0.38×s.c×pH+1.00×s. c×i.t-1.38×pH×i.t. So, the model is considered for prediction within the reach of multiple employed. For gaining better understanding of variables effects on the production of TPC, the predicted model is represented as 3D response surface graphs (fig. 9a-fig. 9c).
The weed C. rotundus can be useful for pharmaceutical industries to make therapeutic herbal drugs since it is rich in tannins. The antioxidant properties of the plant can be useful to reduce oxidative stress in human and animals. Further, it can be grown easily in different types of climatic conditions varied from dried to moist soils and shows irresistible growth. The stem and flower parts of the plant possess high quantity of secondary metabolites in compared to flower. The therapeutic potential of phenolics from different source has been reported. Here in this study, we have extracted phenolics by different conventional and modern techniques. Out of various extraction methods, the MAE finds to show high yield (18.74 %) and phenolic value (118.80 mg/g) in 4 min and 6 min of incubation time with water solvent. In comparison to other methods, MAE takes minimum time to produce the satisfactory results. So, it can be concluded that the new non-conventional methods are more efficient and less time consuming. Now days, RSM technique has also been widely used to optimize secondary metabolites in the large-scale production of bioactive compounds. We have reported the economical and fastest method for phenolics, and other secondary metabolite extraction based on experimental data. In future, the study will be useful for Nutraceuticals, cosmeceutical, pharmaceutical and research Organisation to isolate and optimize the bioactive compounds and drug discovery.
Acknowledgments:
The authors are obliged to Vice Chancellor, of Banasthali Vidyapith for providing all necessary support. We also acknowledgment the Bioinformatics Centre, Banasthali vidyapith supported by DBT and DST for providing computation and networking support through the FIST and CURIE programs at the Department of Bioscience and Biotechnology, Banasthali Vidyapith, Rajasthan.
Conflict of interests:
The authors declared no conflict of interests.
References
- Savithramma N, Rao ML, Suhrulatha D. Screening of medicinal plants for secondary metabolites. Middle East J Sci Res 2011;8(3):579-84.
- Dey D, Quispe C, Hossain R, Jain D, Ahmed Khan R, Janmeda P, et al. Ethnomedicinal use, phytochemistry, and pharmacology of Xylocarpus granatum J. Koenig. Evid Based Complement Alternat Med 2021;2021.
[Crossref] [Google Scholar] [PubMed]
- Polu PR, Nayanbhirama U, Khan S, Maheswari R. Assessment of free radical scavenging and anti-proliferative activities of Tinospora cordifolia Miers (Willd). BMC Complement Alternat Med 2017;17(1):1-2.
[Crossref] [Google Scholar] [PubMed]
- Hossen SM, Hossain MS, Yusuf AT, Chaudhary P, Emon NU, Janmeda P. Profiling of phytochemical and antioxidant activity of wild mushrooms: Evidence from the in vitro study and phytoconstituent's binding affinity to the human erythrocyte catalase and human glutathione reductase. Food Sci Nutr 2022;10(1):88-102.
[Crossref] [Google Scholar] [PubMed]
- Jain D, Janmeda P. Morphology, anatomy, and histochemistry of leaves, stem, and bark of Gymnosporia senegalensis (Lam.) Loes. Lett Appl Nano Biosci 2022;13(2):33.
- Jain D, Uniyal N, Mitra D, Janmeda P. Traditional resources and use of aromatic and ethnomedicinal plants in Uttarakhand: Compliment of nature. Int J Herbal Med 2020;8(5):88-95.
- Varshney N, Jain D, Janmeda P, Mitra D. Role of medicinal plants in pharmaceutical sector: An overview. Global J Bio Sci Bio Technol 2021;10(2):18-24.
- Batool R, Khan MR, Sajid M, Ali S, Zahra Z. Estimation of phytochemical constituents and in vitro antioxidant potencies of Brachychiton populneus (Schott & Endl.) R. Br. BMC Chem 2019;13:32.
[Crossref] [Google Scholar] [PubMed]
- Kasote DM, Katyare SS, Hegde MV, Bae H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int J Biol Sci 2015;11(8):982-91.
[Crossref] [Google Scholar] [PubMed]
- Singh A, Kulia A, Yadav G, Banerjee R. Polyphenol extraction from Okara, food technol. Biotechnol 2011;49(3):322-8.
- Peerzada AM, Ali HH, Naeem M, Latif M, Bukhari AH, Tanveer A. Cyperus rotundus L.: Traditional uses, phytochemistry, and pharmacological activities. J Ethnopharmacol 2015;174:540-60.
- Kamala A, Middha SK, Gopinath C, Sindhura HS, Karigar CS. In vitro antioxidant potentials of Cyperus rotundus L. rhizome extracts and their phytochemical analysis. Pharmacogn Magazine 2018;14(54):261-7.
[Google Scholar] [PubMed]
- Sharma V. Microscopic studies and preliminary pharmacognostical evaluation of Euphorbia neriifolia L. leaves. Indian J Nat Prod Resour 2013;4(4):348-57.
- Singleton VL, Orthofer R, Lamuela-Raventós RM. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol 1999;299:152-78.
- Stankovic MS. Total phenolic content, flavonoid concentration and antioxidant activity of Marrubium peregrinum L. extracts. Kragujevac J Sci 2011;33(2011):63-72.
- Olajire AA, Azeez L. Total antioxidant activity, phenolic, flavonoid and ascorbic acid contents of Nigerian vegetables. Afr J Food Sci Technol 2011;2(2):22-9.
- Sadasivam S, Manickam A. Phenolics. Biochemical methods. New age international (P) publishers: New Delhi; 2004. p. 195-7.
- Makkar HP, Blümmel M, Borowy NK, Becker K. Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J Sci Food Agricul 1993;61(2):161-5.
- Velioglu Y, Mazza G, Gao L, Oomah BD. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agricul Food Chem 1998;46(10):4113-7.
- Ajanal M, Gundkalle MB, Nayak SU. Estimation of total alkaloid in Chitrakadivati by UV-Spectrophotometer. Ancient Sci Life 2012;31(4):198-201.
[Google Scholar] [PubMed]
- Simee W. Isolation and determination of antinutritional compounds from root to shells of peanut (Arachis Hypogaea). J Disper Sci Technol 2011;28:341-7.
- Jain D, Shrivastava S. Estimation of Total Phenolic. Flavonoid and Saponin Content in Different Extracts of Butea monosperma Bark. IJETSR 2017;4(7):177-82.
- Chaudhary P, Janmeda P. Quantification of phytochemicals and in vitro antioxidant activities from various parts of Euphorbia neriifolia Linn. J Appl Biol Biotechnol 2022;10(2):133-45.
- Dayananda B, Raghavan AK, Khanum F, Singh BA. In vitro antioxidant and free radical scavenging activity of Glycyrrhiza glabra root extracts. J Herbal Med Toxicol 2010;4(1):97-102.
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature 1958;181:1199-200.
- Nishikimi M, Rao NA, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 1972;46(2):849-54.
[Crossref] [Google Scholar] [PubMed]
- Payá M, Ferrandiz ML, Miralles F, Montesinos C, Ubeda A, Alcaraz MJ. Effects of coumarin derivatives on superoxide anion generation. Arzneimittelforschung 1993;43(6):655-8.
[Google Scholar] [PubMed]
- UK GM, Gomathi P. Evaluation of antioxidant and free radical scavenging activities of Plumeria acuminata leaves. J Biol Sci 2007;7(2):1361-7.
- Liu T, Li Z, Li R, Cui Y, Zhao Y, Yu Z. Composition analysis and antioxidant activities of the Rhus typhina L. stem. J Pharm Anal 2019;9(5):332-8.
[Crossref] [Google Scholar] [PubMed]
- Dinis TC, Madeira VM, Almeida LM. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophy 1994;315(1):161-9.
[Crossref] [Google Scholar] [PubMed]
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 1996;239(1):70-6.
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal Biochem 1999;269(2):337-41.
[Crossref] [Google Scholar] [PubMed]
- De Castro ML, Garc?a-Ayuso LE. Soxhlet extraction of solid materials: An outdated technique with a promising innovative future. Anal Chim Acta 1998;369(1-2):1-10.
- Chemat F, Tomao V, Virot M. In: Otles S. (Ed.), Handbook of food analysis instruments. Ultrasound-assisted extraction in food analysis. CRC Press: 2008. p. 85-94.
- Kalil SJ, Maugeri F, Rodrigues MI. Response surface analysis and simulation as a tool for bioprocess design and optimization. Proc Biochem 2000;35(6):539-50.
- Rao KJ, Kim CH, Rhee SK. Statistical optimization of medium for the production of recombinant hirudin from Saccharomyces cerevisiae using response surface methodology. Proc Biochem 2000;35(7):639-47.
- Puri S, Beg QK, Gupta R. Optimization of alkaline protease production from Bacillus sp. by response surface methodology. Curr Microbiol 2002;44(4):286-90.
[Crossref] [Google Scholar] [PubMed]
- Vohra A, Satyanarayana T. Statistical optimization of the medium components by response surface methodology to enhance phytase production by Pichia anomala. Proc Biochem 2002;37(9):999-1004.
- Burkert JF, Maugeri F, Rodrigues MI. Optimization of extracellular lipase production by Geotrichum sp. using factorial design. Bioresour Technol 2004;91(1):77-84.
[Crossref] [Google Scholar] [PubMed]
- Bandar H, Hijazi A, Rammal H, Hachem A, Saad Z, Badran B. Techniques for the extraction of bioactive compounds from Lebanese Urtica Dioica. Am J Phytomed Clin Ther 2013;1(6):507-13.
- Pan X, Niu G, Liu H. Microwave-assisted extraction of tea polyphenols and tea caffeine from green tea leaves. Chem Eng Proc 2003;42(2):129-33.
- Proestos C, Komaitis M. Application of microwave-assisted extraction to the fast extraction of plant phenolic compounds. LWT-Food Sci Technol 2008;41(4):652-9.
- Prakash A, Jain D, Tripathi R, Janmeda P. Pharmacognostical analysis of different parts of Cyperus rotundus L. Plant Sci Today 2019;6(1):607-12.
- Bimakr M, Rahman RA, Taip FS, Ganjloo A, Salleh LM, Selamat J, et al. Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves. Food Bioprod Proc 2011;89(1):67-72.
- Prakash A, Janmeda P, Pathak P, Bhatt S, Sharma V. Development and standardization of quality control parameters of different parts of Trianthema portulacastrum L. SN App Sci 2019;1:1-4.
- Sharma M, Bhaskar P. Phenolic compounds in whole-grains of wheat: A review. Appl Biol Chem J 2021;2(1):8-17.
- Krongrawa W, Limmatvapirat S, Pongnimitprasert N, Meetam P, Limmatvapirat C. Formulation and evaluation of gels containing coconut kernel extract for topical application. Asian J Pharm Sci 2018;13(5):415-24.
- Kumaran A, Karunakaran RJ. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT-Food Sci Technol 2007;40(2):344-52.
- Agrawal PK. Carbon-13 NMR of flavonoids. 1st ed. New York: Elsevier; 1989. p. 562-3.
- Miliauskas G, Venskutonis PR, Van Beek TA. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem 2004;85(2):231-7.
- Muthukumaran P, Saraswathy N, Aswitha V, Balan R, Gokhul VB, Indumathi P, et al. Assessment of total phenolic, flavonoid, tannin content and phytochemical screening of leaf and flower extracts from Peltophorum pterocarpum (DC.) Backer ex K. Heyne: A comparative study. Pharmacogn J 2016;8(2).
- Bonjean K, De Pauw-Gillet MC, Defresne MP, Colson P, Houssier C, Dassonneville L, et al. The DNA intercalating alkaloid cryptolepine interferes with topoisomerase II and inhibits primarily DNA synthesis in B16 melanoma cells. Biochemistry 1998;37(15):5136-46.
[Crossref] [Google Scholar] [PubMed]
- Ayitey-Smith E, Addae-Mensah I. Phytochemical, nutritional and medical properties of some leafy vegetables consumed by Edo people of Nigeria. W Afr J Pharmacol Drug Res 1977;4:7-8.
- Al-Shammari AM, Abo-Altemen RA, Shawkat MS. Cyperus rotundus L. alkaloid extracts enhance oncolytic Newcastle disease virus against digestive system neoplasms. South Afr J Bot 2021;143:266-73.
- Man S, Gao W, Zhang Y, Huang L, Liu C. Chemical study and medical application of saponins as anti-cancer agents. Fitoterapia 2010;81(7):703-14.
[Crossref] [Google Scholar] [PubMed]
- Anggraini T, Wilma S, Syukri D, Azima F. Total phenolic, anthocyanin, Catechins, DPPH radical scavenging activity, and toxicity of Lepisanthes alata (Blume) Leenh. Int J Food Sci 2019;2019:9703176.
[Crossref] [Google Scholar] [PubMed]
- Aykul S, Martinez-Hackert E. Determination of half-maximal inhibitory concentration using biosensor-based protein interaction analysis. Anal Biochem 2016;508:97-103.
[Crossref] [Google Scholar] [PubMed]
- Bui LT, Nguyen QT, Dao LM, Nguyen HL, Lam MV, Hoang CT. Evaluation of antimicrobial, antioxidant and cytotoxic activities of Dialium cochinchinensis seed extract. Indian J Pharm Sci 2019;81(5):975-80.
- Saeed N, Khan MR, Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Alter Med 2012;12:1-2.
[Crossref] [Google Scholar] [PubMed]
- Robak J, Gryglewski RJ. Flavonoids are scavengers of superoxide anions. Biochem Pharmacol 1988;37(5):837-41.
[Crossref] [Google Scholar] [PubMed]
- Lim S, Choi AH, Kwon M, Joung EJ, Shin T, Lee SG, et al. Evaluation of antioxidant activities of various solvent extract from Sargassum serratifolium and its major antioxidant components. Food Chem 2019;278:178-84.
- Oscar DÍ, Forero-Doria O, Astudillo L, Gutierrez M, Castro RI, Guzman-Jofre L, et al. Antioxidant activity of bioactive extracts obtained from rhizomes of Cyperus digitatus Roxb. Bol Latinoam Caribe Plant Med Aromát 2014;13(4):344-50.
- Bashir A, Sultana B, Akhtar FH, Munir A, Amjad M, Hassan Q. Investigation on the antioxidant activity of Dheela grass (Cyperus rotundus). Afr J Basic Appl Sci 2012;4(1):1-6.
- Hamed A, Soltan M, Fry J, Hammouda F, Zaki A. Antioxidant and cytoprotective properties of three Egyptian Cyperus species using cell-free and cell-based assays. Pharm Crops 2012;3(1):88-93.
- Luanchoy S, Tiangkul S, Wongkrajang Y, Temsiririrkkul R, Peungvicha P, Nakornchai S. Antioxidant activity of a Thai traditional formula for longevity. Mahidol J Pharm Sci. 2014;41(3):1-5.
- Aeganathan R, Rayar A, Ilayaraja S, Prabakaran K, Manivannan R. Anti-oxidant, antimicrobial evaluation and GC-MS analysis of Cyperus rotundus L. rhizomes chloroform fraction. Am J Ethnomed 2015;2(1):14-20.
- Sathisha AD, Lingaraju HB, Prasad KS. Evaluation of antioxidant activity of medicinal plant extracts produced for commercial purpose. E-J Chem 2011;8(2):882-6.
- Dahmoune F, Spigno G, Moussi K, Remini H, Cherbal A, Madani K. Pistacia lentiscus leaves as a source of phenolic compounds: Microwave-assisted extraction optimized and compared with ultrasound-assisted and conventional solvent extraction. Industrial Crops Prod 2014;61:31-40.
 ): Stem; (
): Stem; ( ): Root and (
): Root and ( ): Flower
): Flower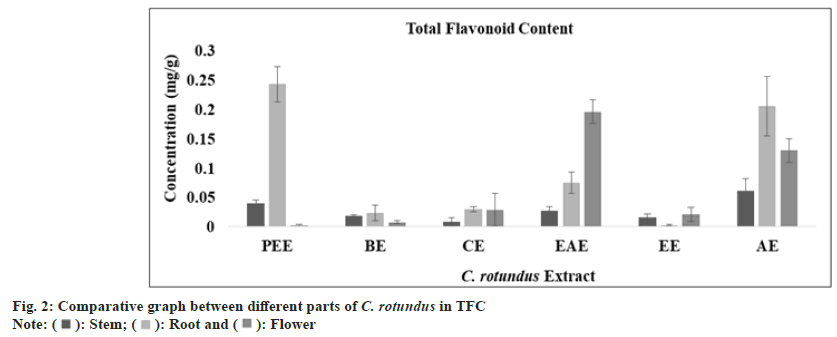
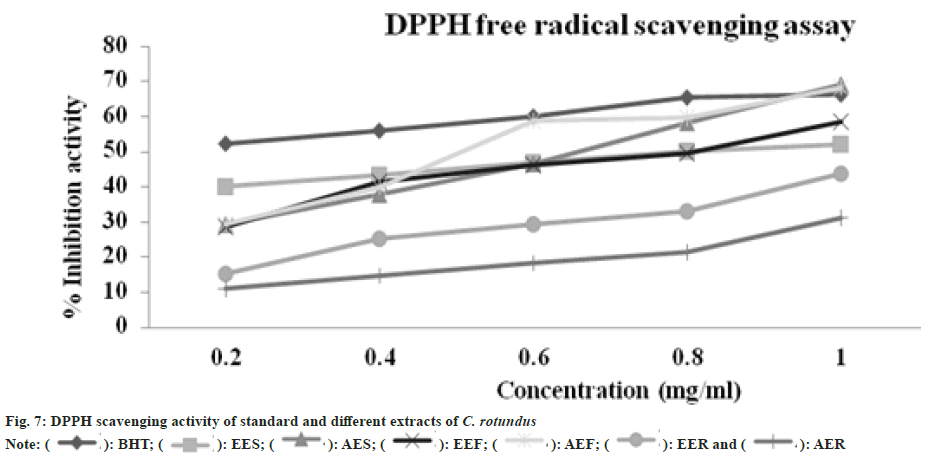
 ): BHT; (
): BHT; ( ): EES; (
): EES; ( ): AES; (
): AES; ( ): EEF; (
): EEF; ( ): AEF; (
): AEF; ( ): EER and (
): EER and ( ): AER
): AER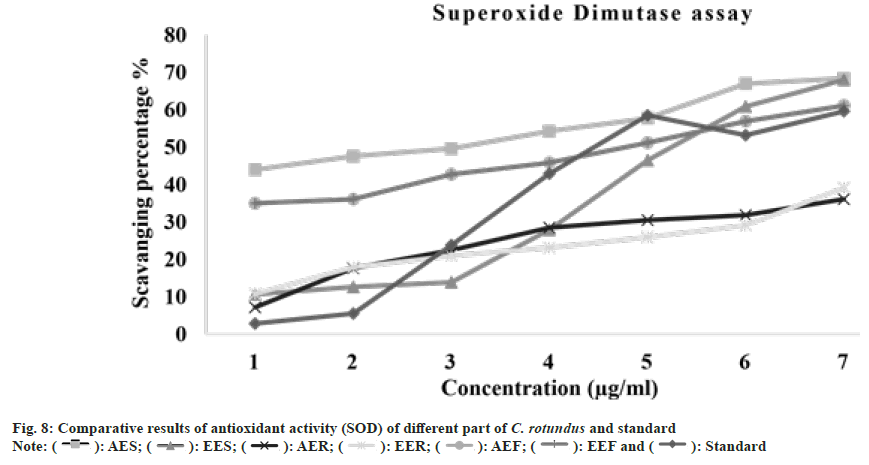
 ): AES; (
): AES; ( ): EES; (
): EES; ( ): AER; (
): AER; ( ): EER; (
): EER; ( ): AEF; (
): AEF; ( ): EEF and (
): EEF and ( ): Standard
): Standard