- *Corresponding Author:
- K. R. Iyer
Department of Pharmaceutical Chemistry, Bombay College of Pharmacy, Kalina, Santacruz (E), Maharashtra 400098, India,
E-mail: krishna.iyer@bcp.edu.in
| Date of Received | 04 September 2022 |
| Date of Revision | 16 April 2023 |
| Date of Acceptance | 19 March 2024 |
| Indian J Pharm Sci 2024;86(2):546-555 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Organic solvents are extensively used in in vitro drug metabolism studies to overcome the solubility issues of lipophilic substrate/new chemical entities. The effects of ten common water miscible organic solvents on cytochrome P450s and non-cytochrome P450 enzymes, namely, flavin monooxygense, aldehyde oxidase, xanthine oxidase, esterases and glutathione S-transferases were evaluated. Rat liver microsomes and rat liver cytosol were used for cytochrome P450, flavin monooxygenase and glutathione S-transferase assays. Partially purified guinea pig liver aldehyde oxidase and rat liver xanthine oxidase fractions were used for molybdenum hydroxylase activity assays. Human plasma was used for esterase activity. Para-nitro phenol, metoprolol, imipramine, methyl para-tolyl sulfide, para-nitrophenyl acetate, vanillin, xanthine and chloro dinitrobenzene, were used as substrates for evaluating CYP2E1, CYP2D6, CYP1A2, flavin monooxyenase, esterase, aldehyde oxidase, xanthine oxidase and glutathione S-transferase activities, respectively. Ten solvents (acetonitrile, acetone, dioxane, dimethyl formamide, dimethyl sulfoxide, ethanol, methanol, polyethylene glycol 400, n-propanol, and isopropanol) were evaluated at four concentrations (0.1, 0.25, 0.5 and 1 % v/v). The following results were obtained. Dioxane (1 % v/v) was found to a potent inhibitor inhibiting all the tested CYP’s (>90 % inhibition) and flavin monooxygenase (71 % inhibition). Seven of ten organic solvents inhibited esterases in a concentration dependent manner out of which n-propanol showed the maximal inhibition (79 % inhibition). Methanol was the only solvent that inhibited aldehyde oxidase (78 % inhibition) and xanthine oxidase mediated metabolism (64 % inhibition). Minimal inhibition effect was observed on glutathione S-transferase mediated activity by all solvents. Polyethylene glycol 400 showed least inhibition of enzyme activities. Among the 10 solvents studies, acetonitrile overall appears to be the safest organic solvent at concentrations of 1 % v/v or less. The results of this study indicate that many water miscible organic solvents must be used very judiciously in drug metabolism studies.
Keywords
In vitro, enzyme inhibition, organic solvent, CYP450, flavin monooxgenase, esterases, aldehyde oxidase, xanthine oxidase, glutathione S-transferase
In vitro drug metabolism studies are well established as tools for the selection of lead molecules at the discovery stage and to evaluate the biotransformation of New Chemical Entities (NCEs)[1-3]. These studies contribute towards the understanding of metabolic stability, metabolic pathways, cross species differences in metabolism, enzyme kinetic parameters, prediction on hepatic clearance and the potential for metabolism based drug-drug interactions[1-3].
The study platforms can be pure enzymes, subcellular fractions (microsomes, cytosol, S9) cells, tissue slices or whole tissue perfusion[1]. These experimental models function optimally only in an aqueous milieu. This requirement presents constrains since a majority of NCEs are lipophilic in nature[1,4]. The poor solubility of NCEs may limit the concentration range that can be studied and, thus, make it difficult to estimate reactions characterized by high apparent Km and low Vmax [5].
Therefore, organic solvents are routinely added as solvents in drug metabolism studies, to enable incubations at higher substrate concentrations[2,3,5]. It is reported that solvents can alter the activities of drug metabolizing enzymes[2,3,5,6-12]. The presence of an organic solvent in an incubation may therefore compromise the reliability and interpretation of in vitro data[3,5,6-12]. Despite several reports on the effects of organic solvent on enzyme activities, according to our estimates, there is no cohesive study that demonstrates the impact of a spectrum of different common organic solvents on the variety of Drug-Metabolizing Enzymes (DME) and tries to delineate the most suitable solvent for the in vitro studies for specific as well as overall drug metabolism studies. Further, most of the studies reported have used lipophilic substrates, and compared the effects of organic solvents to incubation with the least possible amount of solvent. Thus, a true control i.e.,, solvent-free incubation is missing in these reported studies[8,9]. In other studies, substrates were spiked into the incubation vessel, the organic solvent then evaporated and the residue dissolved during microsome addition. It was assumed that complete re-solubilization occurred in the control (without organic solvent) incubation[7].
This study aimed to explore the effects of ten watermiscible organic solvents (Methanol (MeOH), Ethanol (EtOH), n-Propanol (nPA), Isopropanol (IPA), Acetonitrile (ACN), Dimethylsulphoxide (DMSO), Dimethylformamide (DMF), Dioxane (Diox), Polyethylene Glycol 400 (PEG) and Acetone (ACE), on a variety of important DME namely cytochrome P450s (CYP1A2, CYP2D6, CYP2E1), Flavin Monooxygenase (FMO), esterase, Aldehyde Oxidase (AO), Xanthine Oxidase (XO) and Glutathione S-Transferase (GST) activities. In the present study, water-soluble substrates were used to ensure solvent-free incubation and therefore, allowing the true estimation of solvent effects.
Materials and Methods
Imipramine (IMI) and p-Nitrophenol (PNP) was obtained from S. D. Fine-Chem Ltd., Mumbai. PNP was obtained from Lancaster Chemicals. Metoprolol succinate (MET) and Indomethacin was obtained as a gift sample from Ipca Laboratories Ltd., Mumbai. Clomipramine Hydrochloride (HCl) was obtained as a gift sample from Cipla Pvt. Ltd., Mumbai. Methyl p-Tolyl Sulphide (MTS) was obtained from Sigma Ltd., Mumbai. Xanthine was obtained from CDH Laboratory. Vanillin was obtained from Merck, Mumbai. 1-Chloro- 2,4-Dinitrobenzene (CDNB) and p-Nitrophenyl Acetate (p-NPA) was obtained from Himedia Laboratories Ltd., Mumbai. Potassium ferricyanide was obtained from Glaxo Laboratories (India) Ltd., Mumbai. Raloxifene was obtained from Clearsynth, Mumbai. Pindolol and bovine serum albumin were gift samples obtained from Piramal Life Science Pvt. Ltd., Mumbai. Salicylamide, Phenylmethylsulphonyl Fluoride (PMSF), and Nicotinamide-Adenine Dinucleotide Phosphate (NADPH) were obtained from SRL Chemicals Ltd., Mumbai. Tris-HCl and reduced glutathione were obtained from Sigma Chemicals Co., United States of America (USA). Sucrose AR, glycerol, ethylenediamine tetraacetic acid AR, dipotassium hydrogen phosphate AR, Triton X-100, sodium dithionite purified, EtOH AR, Coomassie Brilliant Blue G-250, phosphoric acid (85 % w/v), concentrated HCl, ACE, Diox, nPA, and PEG 400 were obtained from SD Fine Chem Ltd., Mumbai. Calcium chloride AR was obtained from Thomas Baker. Carbon monoxide gas was obtained from Alchemi Gases and Chemicals Pvt. Ltd., Mumbai. ACN, MeOH (High-Performance Liquid Chromatography (HPLC) grade), diethyl ether LR was obtained from Qualigens Ltd., Mumbai, DMF, and DMSO were obtained from Merck India Ltd. All other chemicals were of analytical grade.
Isolation of CYP, FMO, AO, XO and GST containing subcellular fractions:
The rat livers used in this study were obtained from the Department of Pharmacology, Bombay College of Pharmacy, Kalina. Rat livers were obtained from the control animals which were sacrificed as a part of other experiments approved by the Institutional Ethics Committee. Rat Liver Microsomes (RLM) and Rat Liver Cytosol (RLC) fraction were isolated in-house by calcium chloride aggregation method[13] and used for CYP/FMO, and GST assays, respectively. Partially purified AO and XO fractions were isolated from guinea pig and rat livers, respectively, using a reported protocol[14].
Procurement of esterases:
Human plasma was used for esterase activity. There is a marked difference in the plasma hydrolyzing ability between different donors, and thus pooled human plasma was used in the assays[15,16]. Plasma was obtained from King Edward Memorial Hospital, Parel, Mumbai.
Characterization of enzyme pr eparations:
RLM was characterized for spectral CYP450 content by the method of Omura et al.[17]. The protein content was determined by either Bradford method for CYP[18], or Biuret method using Liquixx total protein kit containing reagents and protein standard (bovine serum albumin 6 g/dl) for FMO, AO, XO, esterases and GST. Enzyme fractions were stored at -70° until use.
Enzyme assays:
The incubation conditions for studying the effects of ten different water-miscible organic solvents on different DME are described below and summarized in Table 1.
| Enzyme | Substrate | Substrate (mM) | Cofactor (mM) | Protein (mg/ml) | Time (min) | Quench agent | Incubate volume (ml) | Internal standard |
|---|---|---|---|---|---|---|---|---|
| CYP1A2 | IMI | 25 µM | NADPH | 8.43 | 30 | 12.5 % Ammonia | 0.5 | Clomipramine |
| CYP2D6 | Metoprolol | 25 µM | NADPH | 8.43 | 30 | 6 % Perchloric acid | 1 | Pindolol |
| CYP2E1 | p-Nitro phenol | 50 µm | NADPH | 8.43 | 30 | 0.6 M Perchloric acid | 0.5 | Salicylamide |
| FMO | MTS | 300 µM | NADPH | 20.32 | 45 | 0.5 ml of ACN | 0.5 | 4-Nitroanisole |
| Esterase | p-NPA | 120 µM | None | 54.3 | 10 | None | 3 | None |
| AO | Vanillin | 30 µM | None | 22.6 | 5 | None | 3 | None |
| XO | Xanthine | 10 µM | None | 0.0232 | 10 | None | 3 | None |
| GST | CDNB | 50 µM | GSH | 13.77 | 6 | None | 3 | None |
Table 1: Brief Description of the Incubation Conditions for the Different Enzyme Activity Assays
| Solvents | Percent solvent (v/v) | Total out of 40 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.10 % | 0. 25 % | 0. 5 % | 1 % | ||||||
| Percent inhibition | Score | Percent inhibition | Score | Percent inhibition | Score | Percent inhibition | Score | ||
| MeOH | -3.5 | 1 | -11.8 | 1 | 3.3 | 1 | 1.9 | 2 | 5 |
| DMSO | 2.1 | 4 | 2.1 | 2 | 10.7 | 2 | -1.7 | 1 | 9 |
| Diox | 51.9 | 10 | 81.4 | 10 | 87.1 | 10 | 93.3 | 10 | 40 |
| PEG 400 | 32.5 | 9 | 40.7 | 9 | 49.2 | 9 | 58.3 | 9 | 36 |
| DMF | 5.4 | 5 | 17.6 | 7 | 25.1 | 6 | 37.7 | 5 | 23 |
| IPA | 11.3 | 7 | 16.9 | 6 | 27.1 | 8 | 45.2 | 7 | 28 |
| ACE | 32.4 | 8 | 40.5 | 8 | 17 | 4 | 17 | 4 | 24 |
| EtOH | 8.2 | 6 | 12.3 | 5 | 26.5 | 7 | 39.6 | 6 | 24 |
| ACN | 0.7 | 2 | 4.4 | 3 | 12.5 | 3 | 9.1 | 3 | 11 |
| nPA | 1.3 | 3 | 10 | 4 | 21.1 | 5 | 48.2 | 8 | 20 |
Table 2: Scores of Solvents Based on Percent Inhibition of CYP1A2 at Different Solvent Concentrations
| Solvents | Percent solvent (v/v) | Total out of 40 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.10 % | 0. 25 % | 0. 5 % | 1 % | ||||||
| Percent inhibition | Score | Percent inhibition | Score | Percent inhibition | Score | Percent inhibition | Score | ||
| MeOH | 13.6 | 9 | 32 | 8 | 36.5 | 7 | 51.6 | 5 | 29 |
| DMSO | 6.8 | 6 | 15 | 6 | 18.3 | 4 | 29 | 2 | 18 |
| Diox | 1.4 | 3 | 54 | 9 | 80.8 | 10 | 92.9 | 10 | 32 |
| PEG 400 | 6.3 | 5 | 13 | 5 | 18 | 3 | 34.9 | 3 | 16 |
| DMF | 3.8 | 4 | 12 | 4 | 25.9 | 5 | 52.3 | 6 | 19 |
| IPA | 7.5 | 7 | 9.4 | 3 | 34.3 | 6 | 69.1 | 8 | 24 |
| ACE | -1.3 | 2 | 2.3 | 1 | 7.1 | 1 | 28.2 | 1 | 5 |
| EtOH | 12.9 | 8 | 24 | 7 | 54.6 | 8 | 62.6 | 7 | 30 |
| ACN | -13.8 | 1 | 2.3 | 2 | 15.1 | 2 | 47.3 | 4 | 9 |
| nPA | 31.2 | 10 | 58 | 10 | 69.5 | 9 | 84.7 | 9 | 38 |
Note: Score gained by each solvent is based on the magnitude of inhibition of CYP2D6 activity at every concentration level. Score 1 is assigned to the solvents showing minimum inhibition and score 10 is assigned to the solvents showing maximum inhibition
Table 3: Scores of Solvents Based on Percent Inhibition of CYP2D6 at Different Solvent Concentrations
| Solvents | Percent solvent (v/v) | Total out of 40 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.10 % | 0. 25 % | 0. 5 % | 1 % | ||||||
| Percent inhibition | Score | Percent inhibition | Score | Percent inhibition | Score | Percent inhibition | Score | ||
| MeOH | 8.9 | 3 | 11.5 | 2 | 24.5 | 3 | 37.7 | 3 | 11 |
| DMSO | 73 | 8 | 85.3 | 9 | 89.6 | 9 | 92.4 | 9 | 35 |
| Diox | 76.2 | 10 | 88.6 | 10 | 93 | 10 | 96.3 | 10 | 40 |
| PEG 400 | 7.9 | 2 | 12.2 | 3 | 12.4 | 2 | 20.8 | 2 | 9 |
| DMF | 73.2 | 9 | 81.5 | 8 | 84.3 | 8 | 88.2 | 8 | 33 |
| IPA | 69.6 | 6 | 79.4 | 7 | 82.7 | 7 | 87.6 | 7 | 27 |
| ACE | 10.2 | 4 | 12.8 | 4 | 37.3 | 4 | 57 | 4 | 16 |
| EtOH | 61.5 | 5 | 66.6 | 5 | 71.2 | 5 | 77.5 | 5 | 20 |
| ACN | -5.7 | 1 | -10.4 | 1 | -1.3 | 1 | -23 | 1 | 4 |
| nPA | 72.8 | 7 | 77 | 6 | 80.2 | 6 | 86.1 | 6 | 25 |
Note: Score gained by each solvent is based on the magnitude of inhibition of CYP2E1 activity at every concentration level. Score 1 is assigned to the solvents showing minimum inhibition and score 10 is assigned to the solvents showing maximum inhibition
Table 4: Scores of Solvents Based on Percent Inhibition of CYP2E1 at Different Solvent Concentrations
CYP1A2 activity-IMI N-demethylase activity:
The incubations were performed in 0.1 M phosphate buffer, pH 7.4 in a total volume of 500 μl. Microsomes (8.43 mg/ml protein) were incubated with 10 μl of 1.25 mM IMI sub stocks containing respective organic solvents concentrations appropriately adjusted in order to achieve 0.1 %, 0.25 %, 0.5 %, and 1 % v/v final concentration in the incubation mixture on dilution (final concentration of IMI was 25 μM). The reaction was initiated by addition of 50 μl of 6 mM NADPH. The incubation mixtures were kept at 37° on a water bath shaker for 30 min. The incubations were terminated by the addition of 2000 μl of ammonia solution (12.5 % v/v) and 30 μl of internal standard (clomipramine, 1 mM) was added. The incubations were then extracted using 5 ml diethyl ether. After vortexing the mixture, the two layers (i.e., aqueous and ether) were allowed to separate. The test tubes were then placed in dry ice-ACE bath for 20 s, thereby allowing the bottom aqueous layer to freeze, and upper ether layer was decanted. The ether layer was evaporated on a water bath at 40°. The residue obtained was reconstituted in 200 μl of the mobile phase and then injected to HPLC for analysis. The analysis was performed on Thermo Hypersil BDS, C18 (4.6 mm×250 mm column, 5 μm) at 30°. The mobile phase was composed of ACN; 5 % v/v triethylamine pH 3.0 adjusted with orthophosphoric acid (65:35). The flow rate was 1.0 ml/min with detection at 252 nm. The retention times for metabolite, IMI, and clomipramine were 6.2, 6.9, and 10.7 min, respectively. Control incubations were performed (i.e., in absence of solvent) and each experiment was conducted in triplicate.
CYP2D6 activity-MET hydroxylase activity and O-demethylation:
The incubations were performed in 0.1 M phosphate buffer, pH 7.4 in a total volume of 1000 μl. Microsomes (8.43 mg/ml protein) were incubated with 20 μl of 1.25 mM MET sub stocks containing respective organic solvents concentrations appropriately adjusted in order to achieve 0.1 %, 0.25 %, 0.5 %, and 1 % v/v final concentration in the incubation mixture on dilution (final concentration of MET was 25 μM). The reaction was initiated by addition of 100 μl of 6 mM NADPH. The incubation mixtures were kept at 37° on a water bath shaker for 30 min. The incubations were terminated by the addition of 200 μl of perchloric acid (6 % v/v) and 100 μl of internal standard (pindolol, 0.4 mg/ml) was added. The incubations were then centrifuged at 7000 ×g for 10 min and the supernatant was injected to HPLC for analysis. The analysis was performed on the Jasco HPLC instrument equipped with PU2080 plus HPLC pump, manual Rheodyne injector with a fluorescence detector (FP2080 plus) using Thermo Hypersil BDS, C18 (4.6 mm×250 mm column, 5 μm) at 30°. The mobile phase was composed of ACN; 1 % v/v triethylamine pH 3.0 adjusted with orthophosphoric acid (10:90). The flow rate was 1.0 ml/min with detection at excitation and an emission wavelength of 228 nm and 310 nm, respectively. The retention times for metabolite 1, metabolite 2, pindolol and MET were 5.6, 6.3, 11.1, and 28 min, respectively. Control incubations were performed (i.e., in absence of solvent) and each experiment was conducted in triplicate[3].
CYP2E1 activity para-nitrophenol hydroxylase activity:
The incubations were performed in 0.1 M phosphate buffer, pH 7.4 in a total volume of 500 μl. Microsomes (8.43 mg/ml protein) were incubated with 10 μl of 2.5 mM PNP sub stocks containing respective organic solvents concentrations appropriately adjusted in order to achieve 0.1 %, 0.25 %, 0.5 %, and 1 % v/v final concentration in the incubation mixture on dilution (final concentration of PNP was 50 μM). The reaction was initiated by addition of 50 μl coof 6 mM NADPH. The incubation mixtures were kept at 37° on a water bath shaker for 30 min. The incubations were terminated by the addition of 250 μl of perchloric acid (0.6 M) and 50 μl of internal standard (salicylamide, 350 μg/ml) was added. To this 500 mg of ammonium sulfate was added and the incubation was extracted with 4 ml diethyl ether. After vortexing the mixture, the two layers (i.e., aqueous and ether) were allowed to separate. The test tubes were then placed in dry ice-ACE bath for 20 s, thereby allowing the bottom aqueous layer to freeze, and upper ether layer was decanted. The ether layer was evaporated on a water bath at 40°. The residue obtained was reconstituted in 200 μl of the mobile phase and then injected to HPLC for analysis. The analysis was performed on Thermo Hypersil BDS, C18 (4.6 mm×250 mm column, 5 μm) at 30°. The mobile phase was composed of CAN; 0.1 % formic acid (35:65). The flow rate was 1.0 ml/min with detection at 345 nm. The retention times for salicylamide, p-nitrocatechol (PNC, metabolite), and PNP were 4.99, 5.74, and 8.63 min, respectively. Control incubations were performed (i.e., in absence of solvent) and each experiment was conducted in triplicate[19].
FMO activity-methyl p-tolyl sulfide sulfoxidation activity:
The incubations were performed in 0.1 M Tris- HCl buffer, pH 8.5. Microsomes (20.32 mg/ml protein) were preincubated with 50 μl of Triton X-100 (0.1 %) and left on ice for 10 min before the start of the reaction. Next, 25 μl of NADPH (0.5 mM in Tris-HCl buffer) was added. The mixture was preincubated at 37° for 3 min and then 50 μl organic solvent was added from the appropriate stock to achieve 0.1 %, 0.25 %, 0.5 %, 1 %, and 2 % v/v final solvent concentration in the incubation mixture. The reaction was initiated by the addition of 50 μl of Tris-HCl buffer and 300 μl of MTS (300 μM in Tris-HCl buffer). The incubation mixtures were kept at 37° on a water bath shaker for 45 min. The incubations were terminated by the addition of 250 μl of ACN and 100 μl of internal standard (4-nitroanisole, 1 μg/ml) was added and centrifuged at 13 000 ×g for 10 min. The supernatant was then injected onto HPLC for analysis. The analysis was performed on HiQ Sil C18 (4.6 mm×250 mm column, 5 μm) at 30°. The mobile phase was composed of ACN:water (55:45). The flow rate was 1.0 ml/ min with detection at 230 nm. The retention times for methyl p-tolyl sulfoxide, internal standard, and methyl p-tolyl sulfide were 4.04, 9.45, and 20.5 min, respectively. Control incubations were performed (i.e., in absence of solvent) and each experiment was conducted in triplicate[20].
Esterase activity-para-nitrophenol acetate hydroxylase activity:
The plasma fraction (25 μl) was incubated with 120 μM p-NPA in 0.1 M Tris-HCl buffer containing 0.1 M Calcium chloride (CaCl2) and 100 μl organic solvent containing mix was added from the appropriate stock solutions to achieve 0.1 %, 0.25 %, 0.5 %, 1 % and 2 % v/v final solvent concentration in the incubation mixture, at ambient temperature. The rate of hydrolysis to PNP was measured by an increase in absorbance at 400 nm over a time course of 10 min using continuous spectrophotometry assay method. The instrument used was Jasco (Model No V-530); spectrums were analyzed using spectra manager. The concentration of PNP formed was obtained by dividing the absorbance values with its molar extinction coefficient (10.9 mM-1/cm-1). This data was further mathematically treated to calculate the velocity of the reaction. Blank readings i.e., without the addition of enzyme were taken for each assay. Control incubations were performed (i.e., in absence of solvent) and each experiment was conducted in triplicate[21].
AO activity-vanillin oxidation activity:
The isolated enzyme fraction of the AO (0.09 mg/ ml protein (25 μl of AO fraction)) was incubated with 30 μM vanillin in 67 mM potassium phosphate buffer saline (Sorenson’s buffer), containing 0.1 mM Ethylenediaminetetraacetic Acid (EDTA) (pH 7), potassium ferricyanide solution (1 mM) and 100 μl organic solvent containing mix was added from the appropriate stock to achieve 0.1 %, 0.25 %, 0.5 %, 1 % and 2 % v/v final solvent concentration in the incubation mixture, at ambient temperature. The time course of reduction of potassium ferricyanide to potassium ferrocyanide was monitored by Ultraviolet (UV) spectrophotometry, by a decrease in absorbance at 420 nm, at 1 min interval for 5 min. The instrument used was Jasco (Model No V-530), spectrums were analyzed using spectra manager. The concentration of potassium ferrocyanide formed was obtained by dividing the absorbance values with its molar extinction coof efficient (1.04 mM-1/cm-1). This data was further mathematically treated to calculate the velocity of the reaction. Blank readings i.e., without the addition of enzymes were taken for each assay. Control incubations were performed (i.e., in absence of solvent) and each experiment was conducted in triplicate[22].
XO activity-xanthine oxidation activity:
The isolated enzyme fraction of the XO (0.0232 mg/ml protein (50 μl of 20 fold diluted XO fraction)) was incubated with 10 μM xanthine in 0.05 M potassium phosphate buffer, containing 0.3 mM EDTA (pH 7.5) and 100 μl organic solvent mixture was added from the appropriate stock to achieve 0.1 %, 0.25 %, 0.5 %, 1 % and 2 % v/v final solvent concentration in the incubation mixture, at ambient temperature, where oxygen acted as the cofactor. The time course of formation of UA was monitored by UV spectrophotometry, by an increase in absorbance at 292 nm, at a 1 min interval for 10 min. The instrument used was Jasco (Model No V-530), spectrums were analyzed using spectra manager. The concentration of UA formed was obtained by dividing the absorbance values with its molar extinction co-efficient (12.86 mM-1/ cm-1). This data was further mathematically treated to calculate the velocity of the reaction. Blank readings i.e., without the addition of enzymes were taken for each assay. Control incubations were performed (i.e., in absence of solvent) and each experiment was conducted in triplicate[23].
GST activity-dichloro nitrobenzene glutathione adduct formation:
Rat liver cytosol (13.77 mg/ml protein (100 μl of 100-fold diluted cytosolic fraction)) was incubated with 50 μM CDNB in 0.1 M potassium phosphate buffer (pH 6.5) and 100 μl organic solvent mix was added from the appropriate stock to achieve 0.1 %, 0.25 %, 0.5 %, 1 % and 2 % v/v final solvent concentration in the incubation mixture, at ambient temperature. The time course of formation of 2,4-Dinitrophenol-S-Glucose Transporter (DNP-S-GLUT) was monitored by UV spectrophotometry, by an increase in absorbance at 340 nm, at 1 min interval for 6 min. The instrument used was Jasco (Model No V-530), spectrums were analyzed using spectra manager. The concentration of DNP-S-GLUT formed was obtained by dividing the absorbance values with its molar extinction co-efficient (7.65 mM-1 /cm-1). This data was further mathematically treated to calculate the velocity of the reaction. Blank readings i.e., without the addition of enzymes were taken for each assay. Control incubations were performed (i.e., in absence of solvent) and each experiment was conducted in triplicate.
Data analysis:
For all HPLC based assays, the metabolite to peak area ratio was calculated for each incubation sample by dividing the peak area obtained for metabolite by the peak area of IS. Further, the extent of inhibition or enhancement in the activity was determined by comparing the ratio of peak areas of metabolite to IS in the test incubations (with organic solvent) to the control incubation (no organic solvent), expressed as a percentage.
% residual activity=B/A×100
Where, A is the ratio in a control condition (solvent-free) and B is the ratio in the presence of an organic solvent.
Percent inhibition of activity was calculated as 100 % residual activity. For all the spectrophotometrybased UV assays, the concentration of the product formed was obtained by dividing absorbance by the molar extinction coefficient. The data was further mathematically calculated to obtain velocity of the reaction by using the following formula:
Velocity (nmoles/min)/mg=concentration of product formed (nmoles/ml)/time (min)×protein concentration (mg/ml)
The velocities of the incubation mixture in the absence and presence of solvents were compared to measure the extent of alteration in activity (activation or inhibition).
Percent activity remaining was then calculated using the following equation:
Percent activity remaining=velocity of incubation mixture containing solvent/velocity of control solvent free incubation mixture×100
Percent inhibition of activity was calculated as 100 % activity remaining. All the results are represented as a mean of triplicate incubation.
Results and Discussion
The influence of ten water-miscible organic solvents on a variety of important drugmetabolizing enzyme activities was given below.
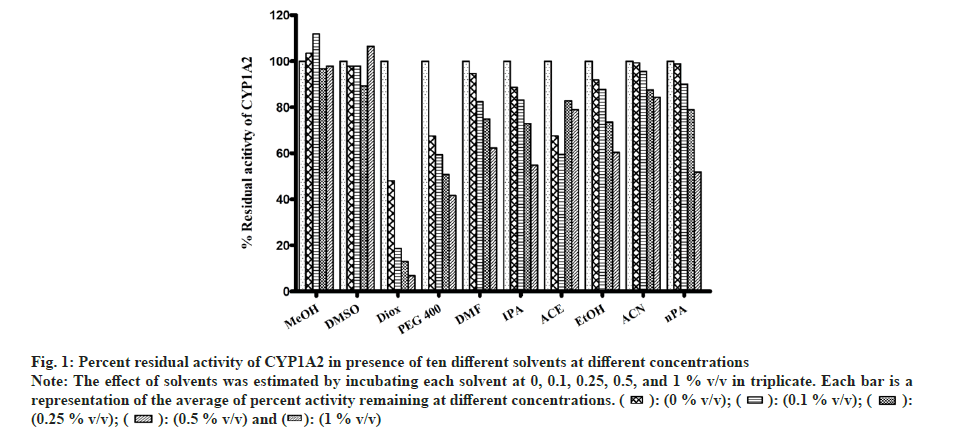
All solvents showed concentration-dependent inhibition except for MeOH and DMSO (fig. 1). Highest inhibitory effect was seen in the presence of Diox (93 % inhibition at 1% v/v concentration). The next highest inhibitory effect was shown by PEG400 (58.6 % inhibition at 1 % v/v level). Remaining solvents had <50 % inhibition at the highest concentration of 1 % v/v.
Fig 1: Percent residual activity of CYP1A2 in presence of ten different solvents at different concentrations
Note: The effect of solvents was estimated by incubating each solvent at 0, 0.1, 0.25, 0.5, and 1 % v/v in triplicate. Each bar is a representation of the average of percent activity remaining at different concentrations. 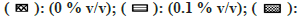
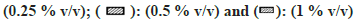
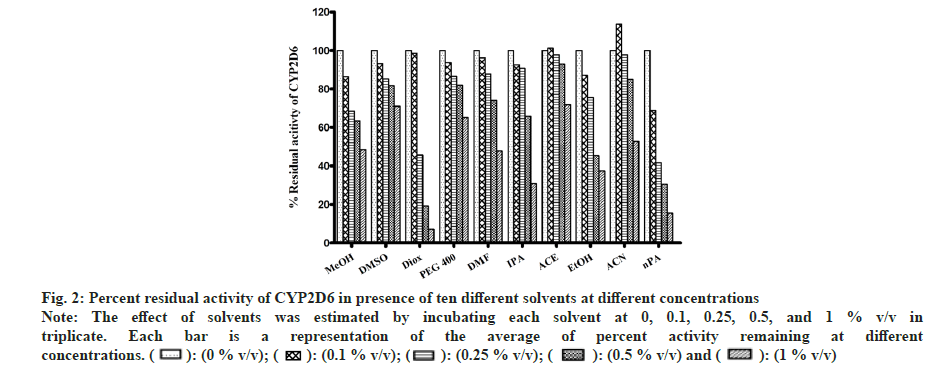
In the case of MET metabolism, all solvents showed concentration-dependent inhibition as depicted in fig. 2. Similar to CYP1A2, Diox showed the greatest inhibition of 92 % at 1 % v/v, followed by nPA showing 84 % inhibition at 1 % v/v. ACE showed the least inhibition of 27 % at 1 % v/v. Few solvents like MeOH, EtOH, IPA, and DMF showed >50 % inhibition, and <50 % inhibition was observed in the case of PEG400, ACE and DMSO. Interestingly, ACN at 0.1% v/v showed activation and increase in activity by 13 % but at 1 % v/v level showed 47 % inhibition (an observation that was consistent when re-evaluated, data not shown).
Fig 2: Percent residual activity of CYP2D6 in presence of ten different solvents at different concentrations
Note: The effect of solvents was estimated by incubating each solvent at 0, 0.1, 0.25, 0.5, and 1 % v/v in triplicate. Each bar is a representation of the average of percent activity remaining at different concentrations. 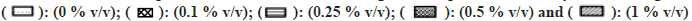
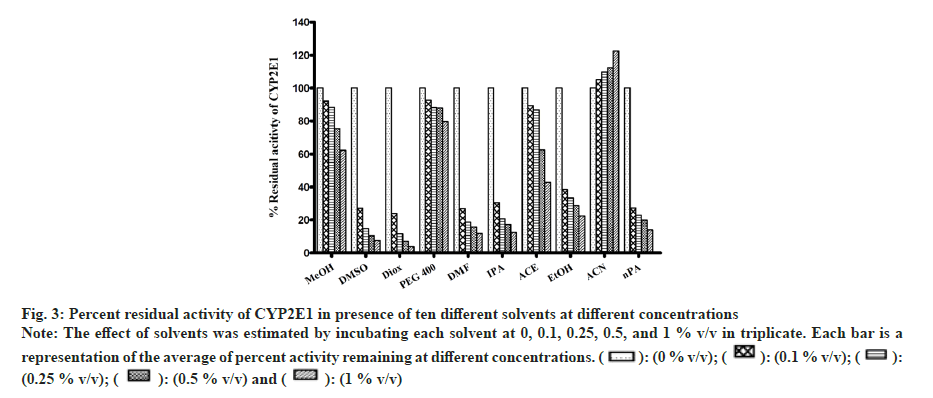
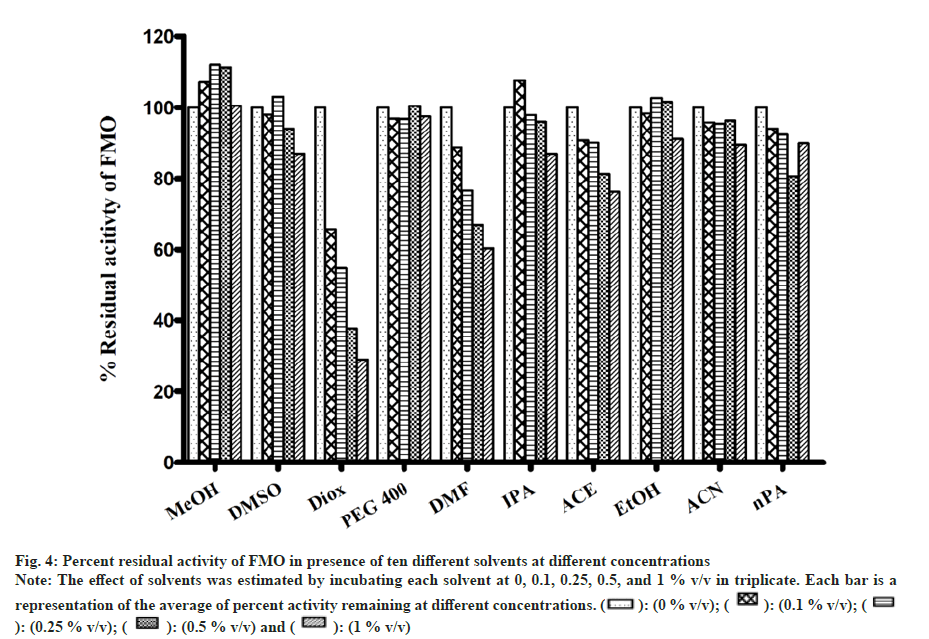
All solvents showed concentration-dependent inhibition except for ACN, which showed activation with an increase in concentration (fig. 3). Among all solvents, Diox showed the maximal inhibition of 96 % at 1 % v/v. High inhibitory effect was also observed with DMSO, DMF, IPA, nPA, EtOH and ACE showing 92 %, 88.1 %, 87.6 %, 86.1 %, 77.5 % and 57 %, respectively, at 1 % v/v. The least inhibition was noticed in the case of MeOH and PEG400 (<50 % inhibition at 1 % v/v). Unlike CYP activity inhibition, relatively less inhibition of FMO activity was observed (fig. 4). Maximum inhibition was shown by Diox (71 % inhibition at 1 % v/v). In the case of MeOH, a small degree of activation at all four concentrations was observed. DMF and ACE showed moderate inhibition of 39 % and 23 % at 1% v/v, respectively, whereas other solvents showed insignificant inhibition (8 %-12 % at 1 % v/v).
Fig 3: Percent residual activity of CYP2E1 in presence of ten different solvents at different concentrations
Note: The effect of solvents was estimated by incubating each solvent at 0, 0.1, 0.25, 0.5, and 1 % v/v in triplicate. Each bar is a representation of the average of percent activity remaining at different concentrations. 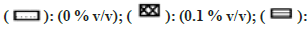
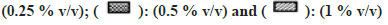
Fig 4: Percent residual activity of FMO in presence of ten different solvents at different concentrations
Note: The effect of solvents was estimated by incubating each solvent at 0, 0.1, 0.25, 0.5, and 1 % v/v in triplicate. Each bar is a
representation of the average of percent activity remaining at different concentrations. 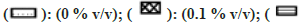
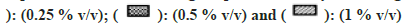
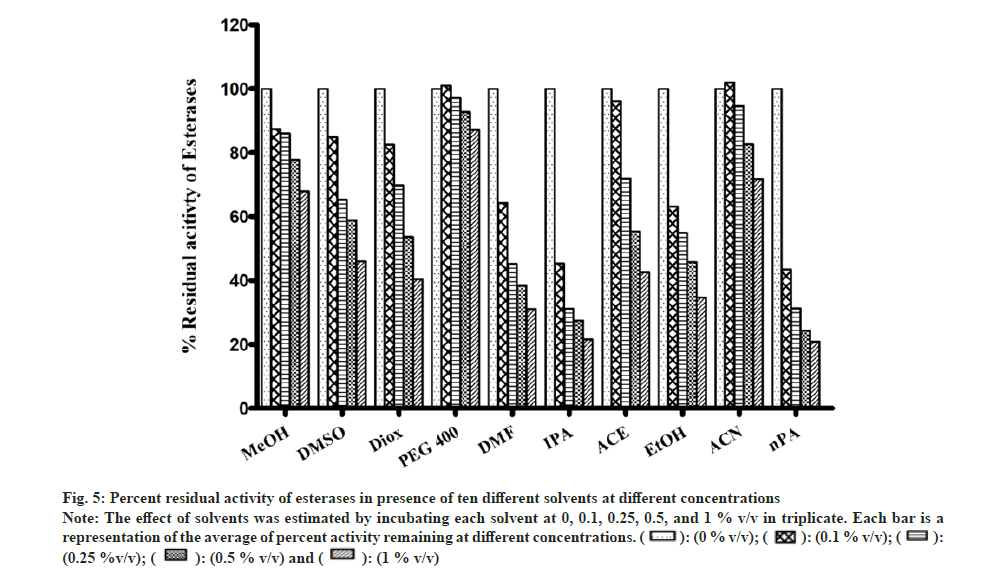
Most of the solvents were found to inhibit the enzyme in a concentration-dependent manner as shown in fig. 5. The esterase activity was inhibited to the highest extent in the presence of nPA (78 % at 1 % v/v). Significant inhibition was observed in the presence of IPA, DMF, EtOH, Diox, and ACE, while the remaining solvents showed minimal inhibition and PEG400 showed almost no effect on the enzyme activity.
Fig 5: Percent residual activity of esterases in presence of ten different solvents at different concentrations
Note: The effect of solvents was estimated by incubating each solvent at 0, 0.1, 0.25, 0.5, and 1 % v/v in triplicate. Each bar is a representation of the average of percent activity remaining at different concentrations. 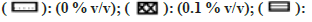
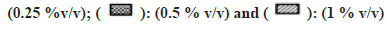
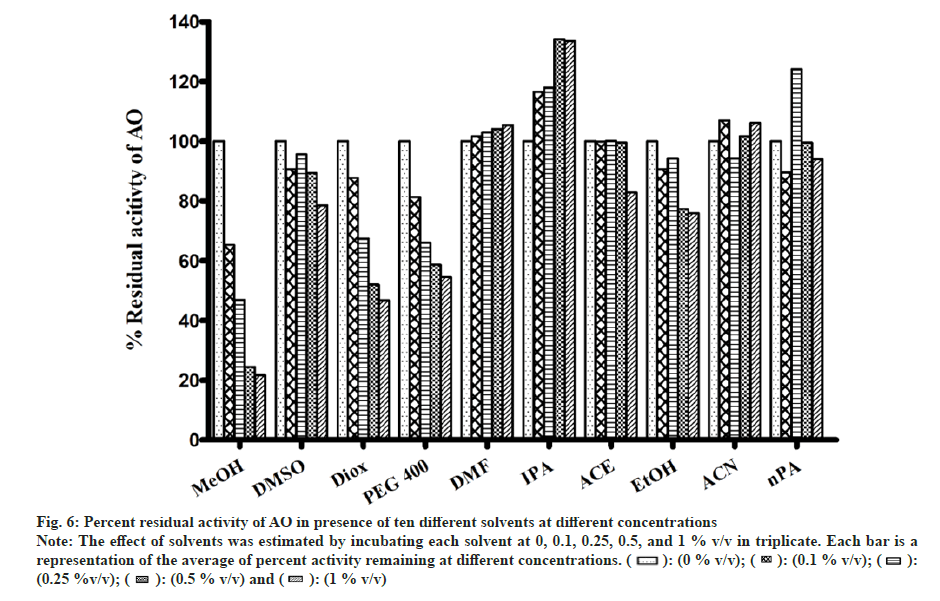
In general, a minor effect on AO activity was seen in the presence of most of the solvents (fig. 6). The only exception was MeOH (78 % inhibition at 1 % v/v) followed by Diox and PEG400 (53 % and 45 % at 1 % v/v). It is important to note that a significant degree of activation was found in the case of IPA at all four concentrations studied (33 % at 1 % v/v).
Fig 6: Percent residual activity of AO in presence of ten different solvents at different concentrations
Note: The effect of solvents was estimated by incubating each solvent at 0, 0.1, 0.25, 0.5, and 1 % v/v in triplicate. Each bar is a representation of the average of percent activity remaining at different concentrations. 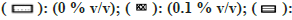
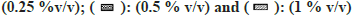
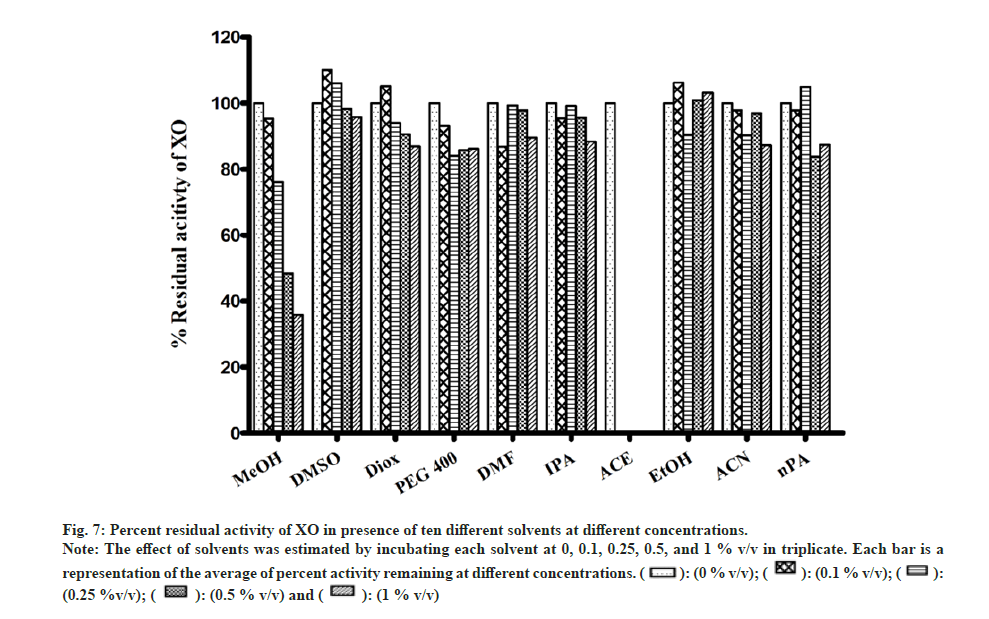
Most of the solvents studied, had minimal inhibitory effect on XO mediated metabolism of xanthine to uric acid (fig. 7). The only exception was MeOH, whose effects were noteworthy (64 % inhibition at 1 % v/v). While performing the assay in the presence of ACE, large fluctuation in absorbance values were obtained. On recording the spectrum of ACE in buffer, it was confirmed that 2 % v/v ACE had an absorbance close to 1, at 292 nm. Hence, the effect of ACE on XO activity could not be studied spectrophotometrically (fig. 7).
Fig 7: Percent residual activity of XO in presence of ten different solvents at different concentrations.
Note: The effect of solvents was estimated by incubating each solvent at 0, 0.1, 0.25, 0.5, and 1 % v/v in triplicate. Each bar is a representation of the average of percent activity remaining at different concentrations. 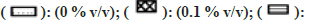
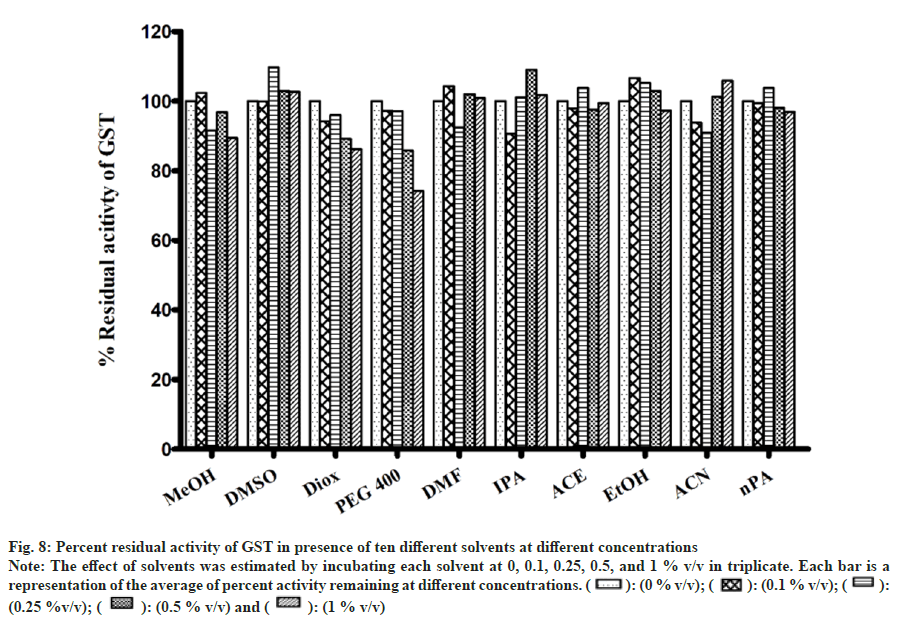
There were no appreciable effects of any of the solvents on GST activity (fig. 8). As a result, metabolism in the presence of solvents remained unaltered. Minimal inhibition was observed with PEG400 of 25 % at 1 % v/v while remaining solvents showed negligible inhibition. Inhibition of GST was only observed when solvent concentration was increase to unrealistic levels of 5 %-20 % v/v (data not shown).
Fig 8: Percent residual activity of GST in presence of ten different solvents at different concentrations
Note: The effect of solvents was estimated by incubating each solvent at 0, 0.1, 0.25, 0.5, and 1 % v/v in triplicate. Each bar is a representation of the average of percent activity remaining at different concentrations.
Drug metabolism is a crucial component that governs the pharmacokinetic profile of the therapeutically used drugs. This makes it very important to understand the metabolism of any compound/New Molecular Entity (NME) at an early stage to prevent the costly termination of its development due to metabolism-related issues. In vitro methods are very commonly used in the preclinical drug development phase[24,25]. Such in vitro methods involve evaluation of the drug metabolism aspects in an aqueous incubation mixture which is further analyzed using sophisticated analytical instruments.
The DME and the co-factor generally interact/ function well in the aqueous component, whereas the NMEs that act as the substrates for the enzymes are usually lipophilic in nature. For the reaction to proceed it is necessary to deliver the substrate to the enzyme, which can be accomplished by dissolving the lipophilic molecules in water-miscible organic solvents like ACN, DMSO, MeOH, etc. However, DMEs are also known to be sensitive to such solvents used during the in vitro assay. Alterations in the activity may lead to erroneous interpretation of various drug metabolism aspects. Good knowledge of the enzyme with respect to the structure, stability and activity in different solvents condition can offer data which make it easier to select appropriate reaction condition for biotransformation of a NCEs[26].
In order to prevent artifacts, it is necessary to have adequate data that helps in selecting an appropriate solvent and its concentration. As indicated in the introduction there have been several literature reports on the effects of small/low concentrations of solvents used as solvents in in vitro incubations. However, most of these reports have been in relation to CYP enzymes and different investigators have evaluated different solvents and different isoenzymes. As such a comprehensive study of a spectrum of different common organic solvents on the variety of DME is lacking to the best of our knowledge. Further, in most of the studies reported a true zero solvent control i.e., solventfree incubation was not established. By using water soluble substrates for all the enzyme activity tested, we have circumvented this issue.
Different solvents have different physicochemical properties and in turn, interact differently with enzymes. The solvents used in this study include alcohols (MeOH, EtOH, 1-propanol, and 2-propanol), an ether (Diox), an amide (DMF), a sulphoxide (DMSO), a ketone (ACE), a nitrile (CAN) and a polyhydroxy polymer (PEG 400). In addition, four concentrations were studied ranging from 0.1, 0.25, 0.50 to 1 % v/v. These concentrations are usually employed for solubilization of lipophilic substrates and abide by protocols that have been generally reported for the usage of solvents in in vitro assays. In combination with the variety of important DME namely cytochrome P450s (CYP1A2, CYP2D6, CYP2E1), FMO, esterase, AO, XO and GST that have been included in this study, it was felt that an overall assessment of the effects of organic solvents on drug metabolizing enzymes would be more forthcoming
In order, to assess the most suitable solvent and its permissible concentration for in vitro studies for a specific or general enzyme assay, we have assigned scores from 1 to 10 to our data obtained for individual enzyme inhibition data at every concentrations level for every solvent studied. For example, at 0.1 % concentration, a score of 1 was assigned to the solvent showing least inhibition, while a score of 10 was assigned to the solvent showing greatest inhibition and this assignment rule was followed for all concentration studied and all solvents studied. Therefore, a total of 4 would be scored by the solvent showing the least inhibition (1 at each of four concentration levels) and a maximum of 40 would be scored by the solvent showing maximum inhibition (10 at each of four concentration levels) as shown in Table 2-Table 9.
| Solvents | Percent solvent (v/v) | Total out of 40 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.10 % | 0. 25 % | 0. 5 % | 1 % | ||||||
| Percent inhibition | Score | Percent inhibition | Score | Percent inhibition | Score | Percent inhibition | Score | ||
| MeOH | -7.12 | 2 | -12.06 | 1 | -11.35 | 1 | -0.67 | 1 | 5 |
| DMSO | 1.94 | 4 | -3.12 | 2 | 5.92 | 6 | 12.93 | 6 | 18 |
| Diox | 34.24 | 10 | 45.26 | 10 | 62.24 | 10 | 71 | 10 | 40 |
| PEG 400 | 3.1 | 5 | 3.17 | 5 | -0.47 | 3 | 2.45 | 2 | 15 |
| DMF | 10.17 | 9 | 23.43 | 9 | 33.05 | 9 | 39.58 | 9 | 36 |
| IPA | -7.63 | 1 | 2.07 | 4 | 3.95 | 5 | 12.99 | 7 | 17 |
| ACE | 9.2 | 8 | 9.87 | 8 | 18.69 | 7 | 23.7 | 8 | 31 |
| EtOH | 1.65 | 3 | -2.71 | 3 | -1.68 | 2 | 8.86 | 3 | 11 |
| ACN | 4.15 | 6 | 4.46 | 6 | 3.62 | 4 | 10.49 | 5 | 21 |
| nPA | 5.92 | 7 | 7.66 | 7 | 19.33 | 8 | 10.02 | 4 | 26 |
Note: Score gained by each solvent is based on the magnitude of inhibition of FMO activity at every concentration level. Score 1 is assigned to the solvents showing minimum inhibition and score 10 is assigned to the solvents showing maximum inhibition
Table 5: Scores of Solvents Based on Percent Inhibition of FMO at Different Solvent Concentrations
| Solvents | Percent solvent (v/v) | Total out of 40 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.10 % | 0. 25 % | 0. 5 % | 1 % | ||||||
| Percent inhibition | Score | Percent inhibition | Score | Percent inhibition | Score | Percent inhibition | Score | ||
| MeOH | 12.7 | 4 | 14 | 3 | 22.2 | 3 | 32.1 | 3 | 13 |
| DMSO | 15.2 | 5 | 34.7 | 6 | 41.2 | 4 | 54 | 4 | 19 |
| Diox | 17.5 | 6 | 30.2 | 5 | 46.4 | 6 | 59.6 | 6 | 23 |
| PEG 400 | -1 | 2 | 2.8 | 1 | 7.3 | 1 | 13 | 1 | 5 |
| DMF | 35.8 | 7 | 54.8 | 8 | 61.6 | 8 | 68.9 | 8 | 31 |
| IPA | 54.7 | 9 | 68.8 | 10 | 72.5 | 9 | 78.5 | 9 | 37 |
| ACE | 3.8 | 3 | 28.1 | 4 | 44.7 | 5 | 57.4 | 5 | 17 |
| EtOH | 36.9 | 8 | 45.2 | 7 | 54.3 | 7 | 65.3 | 7 | 29 |
| ACN | -2 | 1 | 5.4 | 2 | 17.4 | 2 | 28.2 | 2 | 7 |
| nPA | 56.6 | 10 | 68.7 | 9 | 75.6 | 10 | 79.1 | 10 | 39 |
Note: Score gained by each solvent is based on the magnitude of inhibition of plasma esterases activity at every concentration level. Score 1 is assigned to the solvents showing minimum inhibition and score 10 is assigned to the solvents showing maximum inhibition
Table 6: Scores of Solvents Based on Percent Inhibition of Plasma Esterases at Different Solvent Concentrations
| Solvents | Percent solvent (v/v) | Total out of 40 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.10 % | 0. 25 % | 0. 5 % | 1 % | ||||||
| Percent inhibition | Score | Percent inhibition | Score | Percent inhibition | Score | Percent inhibition | Score | ||
| MeOH | 34.79 | 10 | 53.11 | 10 | 75.55 | 10 | 78.39 | 10 | 40 |
| DMSO | 9.38 | 6 | 4.42 | 5 | 10.69 | 6 | 21.46 | 6 | 23 |
| Diox | 12.23 | 8 | 32.57 | 8 | 47.97 | 9 | 53.33 | 9 | 34 |
| PEG 400 | 18.65 | 9 | 33.96 | 9 | 41.42 | 8 | 45.51 | 8 | 34 |
| DMF | -1.6 | 3 | -2.9 | 3 | -4.1 | 2 | -5.4 | 3 | 11 |
| IPA | -16.6 | 1 | -17.9 | 2 | -34.2 | 1 | -33.6 | 1 | 5 |
| ACE | 0.09 | 4 | -0.1 | 4 | 0.38 | 4 | 17.17 | 5 | 17 |
| EtOH | 9.35 | 5 | 5.82 | 7 | 22.64 | 7 | 24.01 | 7 | 26 |
| ACN | -6.9 | 2 | 5.72 | 6 | -1.7 | 3 | -6.1 | 2 | 13 |
| nPA | 10.53 | 7 | -24.1 | 1 | 0.41 | 5 | 6.02 | 4 | 17 |
Note: Score gained by each solvent is based on the magnitude of inhibition of AO activity at every concentration level. Score 1 is assigned to the solvents showing minimum inhibition and score 10 is assigned to the solvents showing maximum inhibition
Table 7: Scores of Solvents Based on Percent Inhibition of AO at Different Solvent Concentrations
| Solvents | Percent solvent (v/v) | Total out of 40 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.10 % | 0. 25 % | 0. 5 % | 1 % | ||||||
| Percent inhibition | Score | Percent inhibition | Score | Percent inhibition | Score | Percent inhibition | Score | ||
| MeOH | 4.72 | 7 | 23.88 | 9 | 51.6 | 9 | 64.18 | 9 | 34 |
| DMSO | -10.1 | 1 | -6.05 | 1 | 1.8 | 2 | 4.18 | 2 | 6 |
| Diox | -5.1 | 3 | 5.97 | 5 | 9.53 | 6 | 13.08 | 7 | 21 |
| PEG 400 | 6.92 | 8 | 15.98 | 8 | 14.14 | 7 | 13.73 | 8 | 31 |
| DMF | 13.26 | 9 | 0.64 | 3 | 2.17 | 3 | 10.5 | 3 | 18 |
| IPA | 4.59 | 6 | 0.79 | 4 | 4.34 | 5 | 11.68 | 4 | 19 |
| ACE | 0 | ||||||||
| EtOH | -6.3 | 2 | 9.73 | 6 | -0.91 | 1 | -3.14 | 1 | 10 |
| ACN | 2.17 | 5 | 9.82 | 7 | 3.06 | 4 | 12.79 | 6 | 22 |
| nPA | 2.15 | 4 | -4.95 | 2 | 16.18 | 8 | 12.63 | 5 | 19 |
Note: Score gained by each solvent is based on the magnitude of inhibition of XO activity at every concentration level. Score 1 is assigned to the solvents showing minimum inhibition and score 10 is assigned to the solvents showing maximum inhibition
Table 8: Scores of Solvents Based on Percent Inhibition of XO at Different Solvent Concentrations
Further, we have computed the gross effect of these water-miscible organic solvents obtained from all enzymes, by adding a score of each organic solvent on all the activities studied. In totality, eight enzymes were studied, covering CYP and non-CYP enzymes. Thus, the best organic solvent would score 32 out of 320 (least score i.e., 4 in each activity and multiplied by 8) and the worst solvent would score 320 out of 320 (maximum score 40 in each activity and multiplied by 8) as shown in Table 10. Such a table is of great utility and value in guiding the selection of a suitable solvent for overall in vitro studies or specific enzyme-mediated metabolism. The overall order of the suitability of different solvents across the different enzyme activities evaluated, from best to worst, was ACN>ACE>DMSO>EtOH>MeOH> IPA>PEG>DM F>nPA>Diox.
| Solvents | Percent solvent (v/v) | Total out of 40 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.10 % | 0. 25 % | 0. 5 % | 1 % | ||||||
| Percent inhibition | Score | Percent inhibition | Score | Percent inhibition | Score | Percent inhibition | Score | ||
| MeOH | -2.4 | 3 | 8.5 | 9 | 3.2 | 8 | 10.58 | 8 | 28 |
| DMSO | 0.1 | 4 | -9.7 | 1 | -3 | 2 | -2.7 | 2 | 9 |
| Diox | 5.8 | 8 | 4 | 7 | 10.8 | 9 | 13.8 | 9 | 33 |
| PEG 400 | 2.7 | 7 | 2.8 | 6 | 14.2 | 10 | 25.8 | 10 | 33 |
| DMF | -4.3 | 2 | 7.6 | 8 | -2 | 4 | -0.9 | 4 | 18 |
| IPA | 9.4 | 10 | -1.1 | 5 | -9 | 1 | -1.7 | 3 | 19 |
| ACE | 2.1 | 6 | -3.8 | 4 | 2.4 | 7 | 0.6 | 5 | 22 |
| EtOH | -6.7 | 1 | -5.3 | 2 | -3 | 3 | 2.6 | 6 | 12 |
| ACN | 6.2 | 9 | 9.2 | 10 | -1.3 | 5 | -5.9 | 1 | 25 |
| nPA | 0.5 | 5 | -3.8 | 3 | 1.81 | 6 | 3.1 | 7 | 21 |
Note: Score gained by each solvent is based on the magnitude of inhibition of GST activity at every concentration level. Score 1 is assigned to the solvents showing minimum inhibition and score 10 is assigned to the solvents showing maximum inhibition
Table 9: Scores of Solvents Based on Percent Inhibition of GST at Different Solvent Concentrations
| Solvents | Score in metabolism study | Overall score | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CYP2E1 | CYP2D6 | CYP1A2 | FMO | Esterases | XO | AO | GST | ||
| Score | Score | Score | Score | Score | Score | Score | Score | ||
| MeOH | 11 | 29 | 5 | 5 | 13 | 34 | 40 | 28 | 165 |
| DMSO | 35 | 18 | 9 | 18 | 19 | 6 | 23 | 9 | 137 |
| Diox | 40 | 32 | 40 | 40 | 23 | 21 | 34 | 33 | 263 |
| PEG 400 | 9 | 16 | 36 | 15 | 5 | 31 | 34 | 33 | 179 |
| DMF | 33 | 19 | 23 | 36 | 31 | 18 | 11 | 18 | 189 |
| IPA | 27 | 24 | 28 | 17 | 37 | 19 | 5 | 19 | 176 |
| ACE | 16 | 5 | 24 | 31 | 17 | 0 | 17 | 22 | 132 |
| EtOH | 20 | 30 | 24 | 11 | 29 | 10 | 26 | 12 | 162 |
| ACN | 4 | 9 | 11 | 21 | 7 | 22 | 13 | 25 | 112 |
| nPA | 25 | 38 | 20 | 26 | 39 | 19 | 17 | 21 | 205 |
Table 10: Overall Scores of Solvents Based on Percent Inhibition of Different Enzymes at Different Solvent Concentrations
Note: The score gained by each solvent is based on the overall magnitude of inhibition of an enzyme activity at four different concentration levels. Score 4 is assigned to the solvents showing minimum inhibition and score 40 is assigned to the solvents showing maximum inhibition
Proteins are known to be unstable in organic/ aqueous mixtures. Enzymes, which are highly specialized proteins with an extraordinary catalytic ability[27], are no exception to this. Water plays a vital role in maintaining the structure of a protein and in catalysis. The nature of the solvents can severely affect the architecture of protein. It is believed that proteins in non-polar solvents, hold their native structure and are active. On the contrary, proteins in polar solvents, disrupt the structure of proteins and lead to their denaturation. Polar solvents can effortlessly strip off the water from the protein surface and try to form hydrogen bonds with protein atoms leading unfolding of the protein structure[28]. Therefore, MeOH is widely used as a quenching agent for various in vitro studies owing to its denaturing property[29]. In vitro enzyme catalytic activity in solvent containing mixtures can also be altered, due to a variety of reasons like change in conformation, active center blockage, competitive metabolism of solvent, limited diffusion, and accessibility of substrate to the enzyme, unfavorable energetics of substrate desolvation, etc.,[30]. In several studies, the catalytic ability of cytochrome P450, a family of isozymes responsible for the metabolism of the majority of the marketed drugs, and few other enzymes are shown to be altered by different types of water miscible solvents used (Table 11)[31-35]. In most cases, the alteration in activity is concentrationdependent i.e., more the solvent used higher is the alteration in activity making it necessary to limit the use of solvents within the acceptable range.
| Enzyme | Reaction monitored | Solvent effects | References |
|---|---|---|---|
| CYP1A2 | Phenacetin O-deethylation | DMSO-inhibition | [31] |
| CYP2A6 | Coumarin 7-hydroxylation | 1 % and higher CAN-inhibition | [6,7] |
| CYP2C8 | Tolbutamide hydroxylation | Methanol and DMSO-inhibition | [8] |
| CYP2C9 | ACN and ACE-induction | [32] | |
| CYP2C19 | S-Mephenytoin 4’-hydroxylation | DMSO-inhibition | [7] |
| DMSO-inhibition | [8] | ||
| CYP2D6 | Dextromethorphan demethylation | DMSO and CAN-inhibition | [6,7] |
| CYP2E1 | Chlorzoxazone 6-hydroxylation | 1 % and higher DMSO-inhibition | [1,2,7,9] |
| 0.1 % and higher MeOH-inhibition | [1,2,7,9] | ||
| EtOH-inhibition | [12] | ||
| p-Nitro phenol | DMSO, DMF and Diox-inhibition | [5] | |
| CYP3A4 | Testosterone 6 β-hydroxylation | 0.1 % and higher DMSO, inhibition | [7,8] |
| UDPGT | 4-Methyl umbelliferyl glucuronide | No significant effect | [8] |
| PST | 4-Methyl umbelliferyl sulfate | [8] | |
| AO and XO | Phenanthridine oxidation | MeOH-inhibition | [33] |
| AO | Vanillic acid | MeOH-inhibition | [34] |
| GST | Benzo[a] pyrene 4,5-oxide | Diox and tetrahydrofuran-inhibition | [35] |
| Styrene oxide and 1,2-Dichloro 4-nitrobenzene conjugation | ACN-inhibition |
Note: A brief review of investigations of solvents effects on drug metabolizing enzyme activities
Table 11: Reported Literature Data of Effects of Solvents on Different Enzymes
This study aimed to provide data that covers a wider variety of enzymes and water-miscible solvents to throw more light into the guidance for careful selection of solvents while performing in vitro studies. At first glance the top 5 solvents appear to be ACN>ACE>DMSO>EtOH>MeOH. However, it should be noted that ACE data is compromised due to technical difficulties while estimating XO inhibition and ACE is rarely used in drug metabolism studies since it is very volatile. Likewise, even though EtOH appears to be a better than MeOH, the relegation of MeOH to the last position is solely due to its unusual ability to inhibit AO and XO (due to interaction at the molybdopterin centre). Overall, it appears that the top 3 solvents for use as solubilizing agents are ACN>DMSO>MeOH and at levels not exceeding 1 % v/v of the incubation mixture.
It is obvious that solvent-free incubations will always be preferred while performing in vitro drug metabolism. However, our studies do indicate that in order to overcome solubility limitations, one can judiciously choose the solvents (and in permissible concentrations) as per the enzyme of interest without hampering its activity. ACN was found to be the safest option as it showed the least inhibition overall whereas Diox was found to be the worst solvent showing maximum inhibition.
Acknowledgments:
The authors are grateful to DST for the DST-FIST support (DST/NSTMIS/05/207-1/2016-2017). Bristol Myers Squibb and BBRC (Biocon BMS Research Centre) for financial assistance to SHK, and AICTE for supporting the M. Pharm students. The authors also wish to thank Mr. Anish Gomatam for assistance and critical evaluation during the preparation of the manuscript.
Conflict of interests:
The authors declared no conflict of interests.
References
- Sinz MW, Iyer KR. The role and practices of drug metabolism in drug discovery and development. Asian Chem Lett 2001;5(2):93-104.
- Iyer KR, Sinz MW. Discovery screening of the in vitro cytochrome P450 inhibitory potency of developmental HIV-protease inhibitors: Comparison to saquinavir, indinavir, nelfinavir, and PNU 140690. Indian J Pharm Sci 2001;63(5):401-7.
- Shah TS, Kamble SH, Patil PG, Iyer KR. Effect of water-miscible organic solvents on CYP450-mediated metoprolol and imipramine metabolism in rat liver microsomes. Indian J Pharm Sci 2015;77(4):382.
[Crossref] [Google Scholar] [PubMed]
- Lewis DF, Jacobs MN, Dickins M. Compound lipophilicity for substrate binding to human P450s in drug metabolism. Drug Discov Today 2004;9(12):530-7.
[Crossref] [Google Scholar] [PubMed]
- Patil PG, Kamble SH, Shah TS, Iyer KR. Effect of water miscible organic solvents on p-nitrophenol hydroxylase (CYP2E1) activity in rat liver microsomes. Indian J Pharm Sci 2015;77(3):283-9.
[Crossref] [Google Scholar] [PubMed]
- Busby WF, Ackermann JM, Crespi CL. Effect of methanol, ethanol, dimethyl sulfoxide, and acetonitrile on in vitro activities of cDNA-expressed human cytochromes P-450. Drug Metab Disposition 1999;27(2):246-9.
[Google Scholar] [PubMed]
- Chauret N, Gauthier A, Nicoll-Griffith DA. Effect of common organic solvents on in vitro cytochrome P450-mediated metabolic activities in human liver microsomes. Drug Metab Dispos 1998;26(1):1-4.
[Google Scholar] [PubMed]
- Easterbrook J, Lu C, Sakai Y, Li AP. Effects of organic solvents on the activities of cytochrome P450 isoforms, UDP-dependent glucuronyl transferase, and phenol sulfotransferase in human hepatocytes. Drug Metab Dispos 2001;29(2):141-4.
[Google Scholar] [PubMed]
- Hickman D, Wang JP, Wang YI, Unadkat JD. Evaluation of the selectivity of in vitro probes and suitability of organic solvents for the measurement of human cytochrome P450 monooxygenase activities. Drug Metab Dispos 1998;26(3):207-15.
[Google Scholar] [PubMed]
- Tang C, Shou M, Rodrigues AD. Substrate-dependent effect of acetonitrile on human liver microsomal cytochrome P450 2C9 (CYP2C9) activity. Drug Metab Dispos 2000;28(5):567-72.
[Google Scholar] [PubMed]
- Wienkers LC, Heath TG. Predicting in vivo drug interactions from in vitro drug discovery data. Nat Rev Drug Discov 2005;4(10):825-33.
[Crossref] [Google Scholar] [PubMed]
- Li D, Han Y, Meng X, Sun X, Yu Q, Li Y, et al. Effect of regular organic solvents on cytochrome P450-mediated metabolic activities in rat liver microsomes. Drug Metab Dispos 2010;38(11):1922-5.
[Crossref] [Google Scholar] [PubMed]
- Walawalkar P, Serai P, Iyer K. Isolation and catalytic competence of different animal liver microsomal fractions prepared by calcium-aggregation method. Indian J Pharm Sci 2006;68(2):262-5.
- Kadam RS, Iyer KR. Isolation of different animal liver xanthine oxidase containing fractions and determination of kinetic parameters for xanthine. Indian J Pharm Sci 2007;69(1).
- Rudakova EV, Boltneva NP, Makhaeva GF. Comparative analysis of esterase activities of human, mouse, and rat blood. Bull Exp Biol Med 2011;152(1):73-5.
[Crossref] [Google Scholar] [PubMed]
- Bahar FG, Ohura K, Ogihara T, Imai T. Species difference of esterase expression and hydrolase activity in plasma. J Pharm Sci 2012;101(10):3979-88.
[Crossref] [Google Scholar] [PubMed]
- Omura T, Sato R. The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J Biol Chem 1964;239(7):2370-8.
[Google Scholar] [PubMed]
- Walker JM. The protein protocols handbook. New Jersey, Humana Press; 1996.
- Kamble SH. Investigation of solvent effects on cyp450 mediated reactions in hepatic microsomes. Thesis Submitted Univ Mumbai 2014.
- Raikuvar K. Potential modulation of flavin mono-oxygenase activity by co-solvents used in drug metabolism studies. Thesis Submitted Univ Mumbai 2016.
- Sankaran S. Evaluation of alterations of esterase activity in presence of organic solvents. Thesis Submitted Univ Mumbai 2016.
- Betgiri S. Evaluation of effect of water-miscible organic solvents on molybdenum hydroxylase activity. Thesis Submitted Univ Mumbai 2016.
- Tanna R. A study on the effect of water miscible organic solvents on enzyme activity. Thesis Submitted Univ Mumbai 2015.
- Tomar T. Studies on the alteration of glutathione S-transferase activity by organic solvents. Thesis Submitted Univ Mumbai 2015.
- Rodrigues AD. Use of in vitro human metabolism studies in drug development: An industrial perspective. Biochem Pharmacol 1994;48(12):2147-56.
[Crossref] [Google Scholar] [PubMed]
- Wang S, Meng X, Zhou H, Liu Y, Secundo F, Liu Y. Enzyme stability and activity in non-aqueous reaction systems: A mini review. Catalysts 2016;6(2):32.
- Nelson DL, Lehninger AL, Cox MM, editors Lehninger-Principles of biochemistry. New York, WH Freeman; 2008.
- Gorman LA, Dordick JS. Organic solvents strip water off enzymes. Biotechnol Bioeng 1992;39(4):392-7.
[Crossref] [Google Scholar] [PubMed]
- Mattos C, Ringe D. Proteins in organic solvents. Curr Opin Struct Biol 2001;11(6):761-4.
[Crossref] [Google Scholar] [PubMed]
- Klibanov AM. Why are enzymes less active in organic solvents than in water? Trend Biotechnol 1997;15(3):97-101.
- Nirogi R, Kandikere V, Bhyrapuneni G, Ponnamaneni RK, Choudary Palacharla R, Manoharan A. Effect of dimethyl sulfoxide on in vitro cytochrome P4501A2 mediated phenacetin O-deethylation in human liver microsomes. Drug Metab Dispos 2011;39(11):2162-4.
[Crossref] [Google Scholar] [PubMed]
- Palamanda J, Feng WW, Lin CC, Nomeir AA. Stimulation of tolbutamide hydroxylation by acetone and acetonitrile in human liver microsomes and in a cytochrome P-450 2C9-reconstituted system. Drug Metab Dispos 2000;28(1):38-43.
[Google Scholar] [PubMed]
- Rashidi MR, Dehghany M, Dehghan G, Jouyban A, Faridi A. Aldehyde oxidase activity and stability in water-miscible organic solvents. Appl Biochem Biotechnol 2013;169(3):901-10.
[Crossref] [Google Scholar] [PubMed]
- Behera D, Pattem R, Gudi G. Effect of commonly used organic solvents on aldehyde oxidase-mediated vanillin, phthalazine and methotrexate oxidation in human, rat and mouse liver subcellular fractions. Xenobiotica 2014;44(8):722-33.
[Crossref] [Google Scholar] [PubMed]
- Aitio A, Bend JR. Inhibition of rat liver glutathione S-transferase activity by aprotic solvents. FEBS Lett 1979;101(1):187-90.