- *Corresponding Author:
- Kondareddypally Nanjundappa Anitha
Department of Pharmacology, Government College of Pharmacy, Rajiv Gandhi University of Health Sciences, Bangalore, Karnataka 560027, India
E-mail: anithakn.res-shs-pharmacy@dsu.edu.in
| Date of Received | 14 January 2022 |
| Date of Revision | 12 April 2022 |
| Date of Acceptance | 11 November 2022 |
| Indian J Pharm Sci 2022;84(6):1429-1443 |
Abstract
Soluble epoxide hydrolase inhibitors have been reported as antihypertensive, anti-inflammatory, antiulcer and anticancer actions and protect the brain, heart and kidney from damage. Exploring soluble epoxide hydrolase inhibition activity of traditional medicinal plants helps to uncover the new target to treat various complications associated with inflammatory mediators. The present study was planned to explore soluble epoxide hydrolase inhibition activity of fifty medicinal plants commonly used in the traditional medicinal system. The dried plant material of each species was grounded into a coarse powder and separately macerated with absolute methanol and ethyl acetate for 7 d. Then the solvents were evaporated and obtained extracts were solubilized in dimethyl sulfoxide (10 mg/ml) and soluble epoxide hydrolase enzyme inhibitory potencies were evaluated using a fluorescent reporting system. Moreover, preliminary phytochemical screening and high-performance thin layer chromatography analysis were carried out for two extracts that showed potent soluble epoxide hydrolase inhibition activities. The results revealed that 10 methanolic extracts and 20 ethyl acetate extracts were potentially effective in suppressing soluble epoxide hydrolase activity with inhibitory concentration value of less than 10 μg/ml. Among the 30 potentially effective plants, four seed extracts (Celastrus paniculatus, Nigella sativa, Wrightia tinctorial, Vernonia anthhelmintica and Embelia ribes), one leaves extracts (Bergera koengii), one rhizome extracts (Curcuma longa) and one root extracts (Vetiveria zizanioides) were common in both methanolic and ethyl acetate extract. In conclusion, the study report suggests that various natural products used in the traditional medicinal system have many promising soluble epoxide hydrolase inhibitors. Methanolic extracts of seeds of Celastrus paniculatus and Nigella sativa have potent soluble epoxide hydrolase inhibition activities. Further research is warranted to identify a greater number of medicinal plants and active principles responsible for soluble epoxide hydrolase inhibition activities
Keywords
Medicinal plants, Ayurveda, traditional medicine, inflammation, soluble epoxide hydrolase
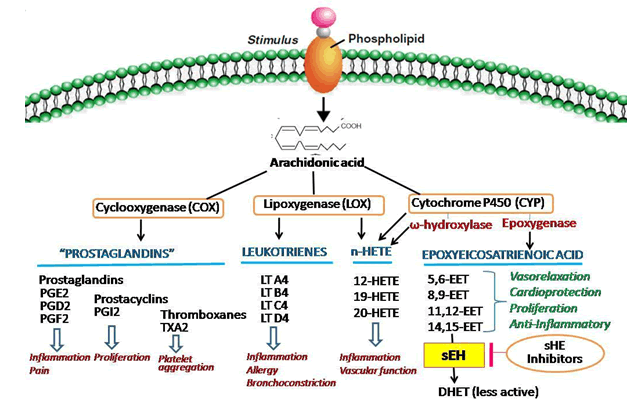
Eicosanoids are implicated in a variety of inflammation-plagued disorders such as atherosclerosis, stroke, hypertension, renal disease, asthma, arthritis, inflammatory bowel disease, ulcers and neurodegenerative disorders[1,2]. Eicosanoids are derived from Arachidonic Acid (ARA) through three pathways; Cyclooxygenase (COX) pathway, Lipoxygenase (LOX) pathway and Cytochrome P450 (CYP) pathway[2,3]. These three pathways of metabolism of ARA to derive various eicosanoids are shown in fig. 1.
The COX pathway yields prostaglandins and thromboxane and LOX pathway yield proinflammatory leukotrienes. The present strategies for treating inflammation and associated complications mainly target these two pathways. Plenty of drugs including non-steroidal antiinflammatory drugs are available in the market and shows antipyretic, analgesic and antiinflammatory actions by inhibiting COX pathway. LOX inhibitors have been used therapeutically in arthritis, seasonal allergies and asthma etc.,[4-6].
The CYP enzyme-mediated eicosanoids synthesis is accompanied by two distinct pathways; the Omega (ω)-hydroxylase and epoxygenase pathways and converts ARA to Hydroxyeicosatetraenoic Acids (HETEs) and Epoxyeicosatrienoic Acids (EETs), respectively[3,6].
EETs are physiologically active compounds that exert various beneficial effects in the body including vasodilation, vascular smooth muscle cell anti-migratory actions, anti-inflammatory actions and suppress hyperthermia, pathological fibrosis, generation of reactive oxygen species, apoptosis, pain and platelet aggregation[1,7-11]. Literature review reveals that EETs also have a role in the modulation of angiogenesis, regulation of cerebral blood flow and mediation of neuroendocrine signaling[12]. Hence, EETs exhibit various beneficial actions like anti-inflammatory, analgesic, antihypertensive, cardio-protective and organ-protective properties.
In mammals, EETs have a very short half-life and the enzyme, soluble Epoxide Hydrolase (sEH) plays a central role in their metabolism[13]. The substrate-specific sEH hydrolyzes EETs to the corresponding Dihydroxyeicosatrienoic Acids (DHETs) whereby the biological effects of EETs are diminished, eliminated or altered[13]. Therefore, inhibiting sEH enzyme increases the half-life EETs and enhances its beneficial properties. Hence, sEH enzyme inhibition is an emerging and promising therapeutic strategy for addressing a variety of diseases[3,13].
Scientific literature survey indicates that various synthetic and natural origin sEH inhibitors have antihypertensive, anti-inflammatory, antiulcer and anticancer actions and protect the brain, heart and kidney from damage[14].
Some of the herbal components like roots of Cimicifuga dahuric[15], Lepidium meyenii and Carica papaya[16] and Sophora flavescens[17] are explored to possess sEH inhibitors with accountable medicinal values. Many herbal products were reported for their anti-inflammatory properties but very less focus was given to the sEH inhibition mediated anti-inflammatory actions[15-17]. Hence, exploring sEH inhibition activity of more traditional medicinal plants helps to uncover a new pharmacological target for old drugs to develop a safe and effective therapeutic strategy from natural sources[18-23].
With the literature knowledge, the present study was planned to explore the sEH inhibition activity of fifty medicinal plants commonly used in Ayurveda. As per our knowledge, the plants selected for this study are not reported for their sEH inhibitory potential. The ethno botanical description of plants used in this study with their Ayurvedic uses and chemical compositions are given in Table 1. Moreover, preliminary phytochemical screening and High-Performance Thin Layer Chromatography (HPTLC) analysis were carried out for two extracts that showed potent sEH inhibition activities.
| Botanical name | Family | Common names | Chemical constitutes | Traditional uses | Reference |
|---|---|---|---|---|---|
| Acalypha indica | Euphorbiaceae | Indian copper leaf | Mauritianin, clitorin, nicrotiflorin, biorobin | Laxative, anthelmintic, emetic, expectorant, to treat scabies, earache, syphilitic ulcers and snake bites etc., | [24] |
| Adhatoda vasica | Acanthaceae | Malabar nut tree | Alkaloids like vasicine, vasicinone, deoxyvasicine, vasicol, adhatodinine, vasicinol | Expectorant, malarial fever, chronic fever, intrinsic haemorrhage, cough, asthma, leprosy, skin diseases, piles | [25] |
| Andrographis paniculata | Acanthaceae | Creat or green chiretta | Diterpenoids, diterpene glycosides, lactones, flavonoids, and flavonoid glycosides | Snakebite, bug bite, diabetes, dysentery, fever, malaria, common cold, diarrhoea, jaundice, as a health tonic etc., | [26] |
| Azadirachta indica | Meliaceae | Neem tree | Gedunin, nimbin, nimbolide, azadirone, neemfruitin etc., | Infection, metabolic diseases, cancer, diabetes mellitus | [27] |
| Bergamia koengii | Rutaceae | Curry Leaf | Vitamin A and calcium | Digestive, tonic, stimulant, diarrhoea, dysentery and vomiting | [27] |
| Cardiospermum halicababum | Sapindaceae | Kanphuti/Ballon plant | Flavones, aglycones, fatty acids, glycosides, terpenoids | Diuretic, demulcent, emetic, laxative, treatment of rheumatism, stiffness of limbs, snakebite | [28] |
| Cassia alata | Fabaceae | Ketepeng | Flavones, flavonols, flavonoids glycosides, alatinon, alanonal and β-sitosterol-β-D-glucoside | Anti-allergic, anti-inflammatory, antioxidant, anticancer, antidiabetic and antifungal | [29] |
| C. paniculatus | Celastraceae | Jyotishmati | Alkaloids, Glycoside, sterols, dipalmitoyl glycerol | Emollient, thermogenic, stimulant, digestive, laxative, emetic, expectorant, appetizer, aphrodisiac, cardiotonic, anti-inflammatory | [30] |
| Centella asiatica | Apiaceae | Indian pennywort, Asiatic pennywort | Saponins, asiaticoside and madecassoside and their aglycones, asiatic acid and madecassic acids | Leprosy, lupus, varicose ulcers, eczema, psoriasis, diarrhoea, fever, amenorrhea | [31] |
| Clerodendrum phlomidis | Lamiaceae | Arni | Sesquiterpene, diterpenoids, triterpenoids, flavonoid and flavonoid glycosides, phenylethanoid glycosides, steroids and steroid glycosides, cyclohexylethanoids, anthraquinones, cyanogenic glycosides | Anti-inflammatory and anti-nociceptive, antioxidant, antihypertensive, anticancer, antimicrobial, anti-diarrheal, hepatoprotective, neuroprotective hypoglycemic, hypolipidemic | [32] |
| Corollocarpus epigaeus | Cucurbitaceae | Jungali suran | Tannins, alkaloids, saponins, steroids and phenolic compound | Laxative, anti-diabetic | [33] |
| Curcuma longa | Zingiberaceae | Termeric | Curcumin, curcumenol, camphor, germacrone, β-pinene, isocurcumenol | Anti-inflammatory, antiviral, anticancerous, carminative, antiproliferative, hypocholesterolemic, diuretic, antidiabetic, antihepatotoxic, antidiarrheal, antirheumatic, hypotensive, antioxidant, antimicrobial, insecticidal, larvicidal, anti-venomous, antithrombotic | [34] |
| Cynodon dactylon | Poaceae | Bermudagrass | Cynodin, hydrocyanic acid and triticin | Anasarca, calculus, cancer, carbuncles, convulsions, cough, cramps, cystitis, diarrhoea, dropsy, dysentery, epilepsy, headache, haemorrhage, hypertension, hysteria, insanity, kidneys, laxative, measles, rubella, snakebite, sores, stones, tumours, urogenital disorders, warts and wounds | [35] |
| Cyperus rotundus | Cyperaceae | Purple nutsedge | Cyperolone, β-cyperone, ρ-cymol, calcium, camphene, copaene, cyperene, cyperenone, cyperol, cyperolone, caryophyllene, cyperotundone, d-copadiene, d-epoxyguaiene, isocyperol, isokobusone, kobusone, limonene, linoleic-acid, linolenic-acid, mustakone, myristic acid, oleanolic acid, oleic acid, β-pinene, patchoulenone, rotundene, rotundenol, rotundone, α-rotunol, β-rotunol, β-selinene, selinatriene, sitosterol, stearic acid, sugeonol and sugetriol | Analgesic, anti-allergic, anti-arthritic, anti-candida, anti-cariogenic, anti-convulsant, anti-diarrheal, anti-emetic, anti-helminthic, anti-hyperglycemic, anti-hypertensive, anti-inflammatory, anti-malarial, anti-obesity, antioxidant, anti-platelet, anti-pyretic, anti-ulcer, anti-viral, ovicidal, gastroprotective, larvicidal, hepatoprotective, neuroprotective, wound healing | [36] |
| Elettaria cardomomum | Zingiberaceae | Cardamom | a- pinene,R- pinene, sabinene, myrcene, limonene, y-terpinene, methyl eugenol | Aromatic stimulant, carminative, stomachic and diuretic | [37] |
| Embelia ribes | Myrsinacae | False black pepper | Embelin, volatile oil, fixed oil, resin, tannin, christembine, caffeic acid, vanillic acid, chrorogenic acid, cinnamic acid, o-cumaric acid | Antibacterial, antifertility activities, antiprotozoal, abdominal disorders, lung diseases, constipation, indigestion, fungus infections, mouth ulcer, sore throat, pneumonia, heart disease, analgesic, anti-inflammatory, antioxidant | [38,39] |
| Glyczirizha glabra | Fabaceae | Liquorice | Liquirtin, isoliquertin liquiritigenin and rhamnoliquirilin | Anti-inflammatory, asthma, expectorant, controls coughing, detoxifies the liver, bronchitis, peptic ulcer, arthritis | [40] |
| Hibiscus rosasinensis | Malvaceae | China rose | Stigmasterol, β-sitosterol, taraxeryl acetate | Cough cold, hair loss, reduction of cholesterol, mild laxative, expectorant and diuretic | [41] |
| Indigofera aspalathoides | Fabaceae | Wiry Indigo | Tannin, alkaloids, triterpenes, flavones, saponin and steroids | Anti-cancerous and antioxidant activity. anti-microbial, hypoglycemic, hepatoprotective, anti-inflammatory and antiviral | [42] |
| Indigofera tinctoria | Fabaceae | True indigo | Alkaloids, flavonoids, tannins and phenols, saponins, glycosides and terpenoids, indigotine, indiruben, rotenoids | Anti-diarrheal, antiviral, antipyretic, antidiabetic, antipsoriatic, antioxidant, antifungal, antineoplastic, antiparasitic, antiseborrheic, anticataract, antithyroid, anticarcinogenic, antileprosy, hair growth stimulant. | [43] |
| Lawsonia alba | Lythraceae | Henna | Lawsone, esculetin, fraxetin, isoplumbagin, scopoletin, betulin, betulinic acid, hennadiol, lupeol, lacoumarin, laxanthone, flavone glycosides, two pentacytic triterpenes | Headache, hemicranias, lumbago, bronchitis, boils, ophthalmia, syphilis, sores, amenorrhea, scabies, dysuria, bleeding disorder, diuretic, antifungal, antibacterial, anti-amoebiasis, astringent, anti-hemorrhagic | [44] |
| Leucas aspera | Lamiaceae | Thumbai | Triterpenoids, oleanolic acid, ursolic acid and b-sitosterol, nicotine, sterols, glucoside, diterpenes, phenolic compounds (4-(24-hydroxy-1-oxo-5-n-propyltetracosanyl)- phenol) | Antifungal, antioxidant, antimicrobial, antinociceptive and cytotoxic | [45] |
| Mentha piperita | Lamiaceae | Peppermint | Menthol, menthyl acetate, menthone, pulegone, menthofuran, limone | Astringent, antiseptic, antipruritic, antiemetic, carminative, vermifuge, diaphoretic, analgestic | [46] |
| Mollugo cerviana | Molluginaceae | Thread stem carpet weed | Carbohydrates, saponins, tannins, terpenoids, flavonoids, steroids, phenols, proteins and alkaloids | Antimicrobial, anti-inflammatory, antioxidant activity and spermicidal activity | [47] |
| N. sativa | Ranunculaceae | Fennel flower | Thymoquinone, thymohydroquinone, dithymoquinone, p-cymene,carvacrol,4-terpineol, t-anethol | Antidiabetic, anticancer, immunomodulator, analgesic, antimicrobial, anti-inflammatory, spasmolytic, bronchodilator, hepatoprotective, renal protective, gastro-protective, antioxidant | [48] |
| Ocimum basilicum | Lamiaceae | Basil | Linalool, methyl chavicol or citral and 1,8-cineole, camphor, thymol, methyl cinnamate, eugenol, methyl eugenol, methyl isoeugenol and elemicine | Headaches, coughs, diarrhoea, constipation, warts, worms and kidney malfunctions | [49] |
| Ocimum sanctum | Lamiaceae | Tulsi | Eugenol, euginal, urosolic acid, carvacrol, linalool, limatrol, caryophyllene, methyl carvicol | Antimicrobial, anti-diarrheal, anti-oxidant, anti-cataract, anti-inflammatory, hepato-protective, analgesic, anti-pyretic, anti-allergic, CNS depressant, anti-asthmatic, anti-tussive, diaphoretic, anti-thyroid, anti-fertility, anti-ulcer, anti-emetic, anti-spasmodic, anti-arthritic, adaptogenic, anti-stress | [50] |
| Phyllanthus emblica | Euphorbiaceae | Amla | tannins, gallic acid, ellagic acid, emblicol, phyllembin, lupeol, essential oil, fixed oil | Antidiabetic, hypolipidemic, antibacterial, antioxidant, antiulcer, hepatoprotective, gastroprotective | [51] |
| Phyllanthus niruri | Euphobiaceae | Stonebreaker | Alkaloid, flavonoid, terpenoids, cardiac glycoside, saponins, tannins, cyanogenic glycosides | Hepatitis B, lithiasis, hyperlipidemia, diabetes, hyperuricemia, nephrotoxicity, platelet aggregation, algesia, unwanted pregnancy, vasoconstriction, hepatotoxicity | [52] |
| Piper betel | Piperaceae | Betel leaf | Terpinine, P-cymene, carvacrol, chavicol and its derivatives, allyl catechol, eugenol, estragol, oxalic acid, malic acid and amino acids. Leaves contain good amounts of vitamins particularly nicotinic acid, ascorbic acid and carotin | Obstructed urination, weakness of nerves, sore throat, respiratory disorders, constipation, problem of breast milk secretion, inflammation, antimicrobial, antifungal, antihistaminic | [53] |
| Piper longum | Piperaceae | Long pepper | Alkaloids, amides, lignans, esters, volatile oils | Cancer, diabetes, obesity, hyperlipidemia, asthma, fungal infection, arthritis, pain, amoebiasis, ulcer, depression, inflammation | [53] |
| Piper nigrum | Piperaceae | Peppercorn | Piperic acid, piperlonguminine, pellitorine, piperolein B, piperamide, piperettine and (-)-kusunokinin | Anti-inflammatory, analgesic, anticonvulsant and neuroprotective, antidiabetic | [54] |
| Plectranthus vettiveroides | Lamiaceae | Hribera | Androstan-17-one 3-ethyl-3-hydroxy-(5α) (-) spathulenol | Antipyretic, diuretic, antibacterial, antioxidant, anticancer, antidiabetic, hepatoprotective | [55] |
| Psoralea corylifolia | Fabaceae | Babchi | Bakuchiol, psoralen, isopsoralen, corylifolin, corylin, psoralidin | Antitumor, antihyperglycemic, antidepressant, antioxidant, antibacterial, diuretic, anthelmintic, laxative, wound healing, stomachic, stimulant, aphrodisiac, diaphoretic, asthma, cough, nephritis | [56] |
| Pungamia glabra | leguminosae | Karanj | Karangin, pongamol, pongagalabrone, and pongapin, pinnatin and kanjone | Antiseptic, anti-inflammatory, anti-plasmodial, anti-nociceptive, anti-hyperglycemic, anti-lipidoxidative, antidiarrhoeal, anti-ulcer, anti-hyperammonic, CNS depressant and antioxidant | [57] |
| Santalum album | Santalaceae | Sandal wood | Alpha-santalol, tannins, terpenes, resins | Anti-inflammatory, antimicrobial, antiproliferative, acne, psoriasis | [57] |
| Sesamum indicum | Pedaliaceae | Benne | sesamol, sesamolin and sesamin | Antioxidants, anti-inflammatory, anti-microbial, anti-pyretic. | [58] |
| Smilax chinensis | Smilacaceae | China root | Beta-sitosterol, caffeic acid, catechin, daucosterin, daucosterol, engeletin, epicatechin, friedelin, heloniosides, hydroxyflavan, isoengeletin, naringenin, piceid, quercetin, resin, resveratrol, rutin, saponin, scirpusin, seiboldogenin, smilacin, smilasides, tannin, taxifolin, trihydroxystibene, vanillic acid, flavonoids and stilbenes | Antimicrobial, anthelmintic, antioxidant, anticancer, hepatoprotective | [59] |
| Solanum trilobatum | Solanaceae | Climbing brinjal | Sobatum,β-solamarine, solasodine,solaine, glycoalkaloid and diosogenin | Hepatoprotective, antimicrobial, antioxidant, cytotoxic, haemolytic, immunomodulatory and anti-inflammatory | [60] |
| Terminalia chebula | Combretaceae | Chebulic myrobalan | Gallic acid, chebulagic acid, punicalagin, chebulanin, corilagin, neochebulinic acid, ellagic acid, chebulinic acid, 1,2,3,4,6-penta-O-galloyl-β-D-glucose, 1,6-di-o-galloyl-D-glucose, casuarinin, 3,4,6-tri-o-glloyl-D-glucose, terchebulin | Antioxidant, antibacterial, antifungal, antiviral, antiprotozoal, antiulcer, anticarcinogenic, purgative, radioprotective, antiallergic. hepatoprotective, anti-inflammatory, antispasmodic | [61] |
| Tinospora cordifolia | Menispermaceae | Guduchi | Berberine, palmatine, isocolumbine, tinocordiside, palmatoside, beta sitosterol, tinosporides | Anti-diabetic, anti-periodic, anti-spasmodic, anti-inflammatory, anti-arthritic, antioxidant, anti-allergic, anti-stress, anti-leprotic, anti-malarial, hepatoprotective, immunomodulatory | [62] |
| Trichosanthes cucumerina | Cucurbitaceae | snake gourd | Proteins, fat, fibre, carbohydrates, minerals, and vitamins A and E in high levels | Antidiabetic, antibacterial, anti-inflammatory, anthelmintic, antifebrile, gastroprotective, and antioxidant activity | [63] |
| Trigonella foenum | Fabaceae | Fenugreek | Diosgenin, trigonelline, fenugreekine, galactomannan and 4-hydroxy isoleucine | In the treatment of diabetes, microbial and cancer disease | [64] |
| Vernonia anthhelmintica | Asteraceae | Purple fleabane | Brassicasterol, stigmasterol, resin, myristic acid palmitic acid, stearic acid, oleic acid linoleic acid vernolic acid and methyl vernolate | Diabetes mellitus, leukoderma, skin disease, fever, worm infection and kidney trouble | [65] |
| Vetiveria zizanioides | Poaceae | Vetiver | Cycloisolongifolene, isoledene, isolongifolene, longifolene, sativene | Antimicrobial | [66] |
| Withania somnifera | Solanaceae | Ashwagandha/winter cherry | Isopelletierine, anaferine, cuseohygrine, anahygrine, withanolides, withaferins | Anti-epileptic, anti-inflammatory, anti-arthritic, anti-depressant, anti-coagulant, antioxidant, anti-diabetic, anti-pyretic | [67] |
| W. tinctoria | Apocynaceae | Sweet Indrajao | Lupeol, α- and β- amyrin, indigotin, indirubin, tryptanthrin, isatin, rutin, β-sitosterol, triacontanol, myristic acid, palmitoleic acid, palmetic acid, stearic acid, behenic acid, arachidic acid | Antidiarrhoeal, aphrodisiac, anthelmintic, febrifuge, stomachic, toothache, tonic and dog bite | [68] |
| Zingiber officinalae | Zingiberaceae | Ginger | Gingerol, diarylheptanoids, glutamate, aspartic acid, serine, glycine, threonine, alanine, cystine, valine, methionine, isoleucine, leucine, tyrosine, phenylalanine, lysine, histidine, arginine, proline | Antioxidant, anti-inflammatory, antimicrobial, anticancer, antiobesity, antidiabetic, antinausea, antiemetic, antiallergic, neuroprotective, hepatoprotective, cardiovascular protective and respiratory protective | [69] |
Table 1: List of Plants used in the Studies with their Details
Materials and Methods
Materials:
The human 5-HETE enzyme-linked immunosorbent assay kit was procured from Fine Test (Fine Biotech, Wuhan, China). Dimethyl Sulfoxide (DMSO), methanol and ethyl acetate were purchased from Hi- Media Laboratories Pvt. Ltd. (Mumbai, India). All other chemicals and reagents used in the study are of analytical grade.
Plant material and extraction procedure:
Plant materials of 50 plant species (Acalypha indica, Adhatoda vasica, Andrographis paniculate, Azadirachta indica, Bergamia koengii, Cardiospermum halicababum, Cassia alata, Celastrus paniculatus (C. paniculatus), Centella asiatica, Clerodendrum phlomidis, Corollocarpus epigaeus, Curcuma longa, Cynodon dactylon, Cyperus rotundus, Elettaria cardomomum, Embelia ribes, Glyczirizha glabra, Hibiscus rosasinensis, Indigofera aspalathoides, Indigofera tinctorial, Lawsonia alba, Lawsonia alba, Leucas aspera, Mentha piperita, Mollugo cerviana, Nigella sativa (N. sativa), Ocimum basilicum, Ocimum sanctum, Phyllanthus emblica (P. emblica), Phyllanthus niruri, Piper betel, Piper longum, Piper nigrum, Plectranthus vettiveroides, Psoralea corylifolia, Pungamia glabra, Santalum album, Sesamum indicum, Smilax chinensis, Solanum trilobatum, Terminalia chebula, Tinospora cordifolia, Trichosanthes cucumerina, Trigonella foenum, Vernonia anthhelmintica, Vetiveria zizanioides, Withania somnifera, Wrightia tinctorial (W. tinctoria) and Zingiber officinalae) included in this study were collected from the local market of Tirupati and authenticated from Department of Botany, Sri Venkateshwara University, Tirupati, India. The voucher specimens were kept in the department herbarium for future reference. Based on traditional and Ayurvedic use mentioned in the literature part of the plant selected for this study. The detailed information about a plant family, chemical constituents, traditional medicinal uses and part of the plant used in the present study is given in Table 1[24-69].
The dried plant materials of each plant were grounded into a coarse powder. 100 g of coarse powder was equally divided into two parts and separately soaked in 200 ml of absolute methanol and 200 ml of ethyl acetate for 7 d. Then the extracts were filtered through double layers of muslin, centrifuged at 7000 rpm for 10 min and finally filtered again through Whatman filter paper no. 41 to attain a clear filtrate. The clear filtrates were evaporated and dried at 40° under reduced pressure using a rotatory vacuum evaporator (LabTech). The extract yields were weighted and stored in glass bottles at 5°.
sEH inhibition activity of plant extracts:
Dried plant extracts were solubilized at 10 mg/ml in DMSO and inhibitory potencies were measured against the human sEH using a fluorescent reporting system (BMG Labtech) as per the method prescribed by Jones et al. Shortly, half maximal Inhibitory Concentration (IC50) values were determined using Cyano (2 Methoxynaphthalen-6-yl) Methyl Trans- (3-phenyl-oxyran-2-yl) Methyl Carbonate (CMNPC) as a fluorescent substrate. Recombinant sEH (1 nM) was incubated with inhibitors for 5 min in 100 mM sodium phosphate buffer (pH 7.4) containing 0.1 mg/ ml of BSA at 30° prior to substrate introduction ((S)=5 μM). The activity was measured by determining the appearance of the 6-methoxy-2-naphthaldehyde with an excitation wavelength of 330 nm and an emission wavelength of 465 nm for 10 min. The DMSO without plant extracts and treated in the same manner as the test was used as a negative control for reference.
Preliminary phytochemical screening of C. paniculatus Methanolic Extract (CPME) and N. sativa Methanolic Extracts (NSME):
Based on the in vitro sEH inhibition activity, two potent extracts: CPME and NSME were evaluated for the following qualitative and quantitative preliminary phytochemical tests.
Qualitative tests: The preliminary qualitative tests for selected extracts (CPME and NSME) were performed for the detection of the presence of alkaloids (Mayer’s test, Dragendorff’s test, Wagner’s test and Hager’s test), carbohydrates (Molisch’s test, Fehling’s test and Benedict’s test), glycosides (Legal’s test and Libermann-Burchard’s), phytosterols (Liebermann-Burchard‘s test), fixed oils and fats (Spot test), saponins (Foam test), phenolic compounds and tannins (ferric chloride test, lead acetate test), proteins and free amino acids (Millon’s test and ninhydrin test), gums and mucilage, flavonoids (Shinoda test, Alkaline reagent test)[18,19].
Quantitative tests: For estimation of total flavonoids, 1 ml of (0.1 % w/v) solution of plant extract in ethanol was mixed with 1 ml Aluminium chloride (AlCl3) (2 % w/v in ethanol) and 1 drop of acetic acid was added to it and made up to 25 ml with distilled water. Similarly, the standard quercetin in 0.1 % w/v in ethanol was treated in the same manner as the sample. The sample and the standard were allowed to stand at room temperature for 40 min. The absorbance was measured at 415 nm and the readings were recorded[18,19]. Total flavonoid content was calculated using the formula shown below.
Total flavanoids=(A×M0)/(A0×M)
Where, A was absorbance of extract, A0 was absorbance of standard, M was weight of extract and M0 was weight of standard.
For the estimation of Total Phenolic Content (TPC), 1 ml of (0.1 % w/v) solution of plant extract in ethanol was mixed with 1.5 ml of Folin Ciocalteu’s reagent and 8.5 ml of water. Allow it to stand for 5 min. Then 4 ml of 20 % sodium carbonate was added in it. The absorbance was measured at 765 nm and TPC was estimated using gallic acid as standard[18,19].
Total alkaloid content was estimated by the gravimetric method. Briefly, a quantity of extract was weighed and transferred to a separating funnel. 10 ml of chloroform was added and the contents were shaken well for 30 min to extract the alkaloids completely. The contents were dried in a china dish and the residue was weighed to calculate the mass of alkaloids[18,19].
HPTLC analysis of CPME and NSME:
HPTLC studies were carried out using a Camag HPTLC system with a Linomat V sample applicator, a Camag 3 TLC scanner and winCATS 4 software for the interpretation of the data. An aluminium plate (20×10 cm) precoated with silica gel 60F254 (E Merck) was used as the adsorbent. The plates were developed using n-hexane:ethyl acetate (5:4) as the mobile phase for plant extracts in a Camag twin trough chamber and scanned at 254, 366 and 425 nm. The Retention factor (Rf) values of the extracts were determined using winCATS 4 software. The developed plates were photo-documented at 254 nm, 366 nm and 425 nm using a Camag 3 Reprostar. The Rf values of extracts were compared with the Rf value of standard Linoleic acid and confirmed by an overlay of spectra.
Statistical analysis:
Prisom software was used for statistical analysis. All the values are expressed in mean±Standard Error of the Mean (SEM). (n=3), Two-way Analysis of Variance (ANOVA) followed by Bonferroni post hoc tests. p<0.05 was considered statistically significant.
Results and Discussion
The extract of 100 g of dried plant materials yielded plant extract residues ranging from 1 % to 25 % with methanol and 0.2 % to 14 % with ethyl acetate. With both the solvents highest extract yield was obtained from fruits of P. emblica while Cynodon dactylon gives the lowest extract yield.
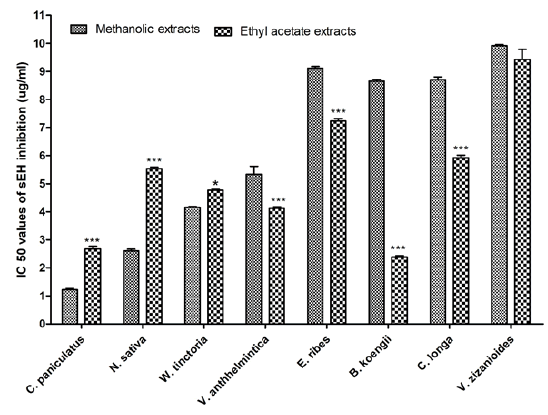
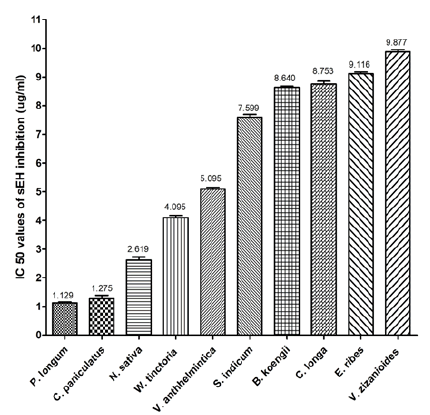
Fifty plant species were investigated to evaluate their sEH enzyme inhibition potential against the human sEH enzyme using a fluorescent reporting system. The results revealed that 10 methanolic extracts and 20 ethyl acetate extracts were potentially effective in suppressing sEH activity with IC50 value of less than 10 μg/ml. The highest potency was observed with methanolic extract of Piper longum fruit (IC50=1.108 μg/ml) but the ethyl acetate extracts of the same were very poor in inhibiting sEH activity (IC50 >50 μg/ ml). In case of ethyl acetate, Bergera koengii leaves extract showed the highest potency with IC50 value of 2.384 μg/ml. Among the 30 potentially effective plants, four seed extracts (C. paniculatus, N. sativa, W. tinctoria, Vernonia anthhelmintica and Embelia ribes), one leaves extracts (Bergera koengii), one rhizome extracts (Curcuma longa) and one root extracts (Vetiveria zizanioides) were common in both methanolic and ethyl acetate extracts. When comparing the sEH inhibition activities of these plants it was observed that methanolic extracts of three plants (C. paniculatus, N. sativa and W. tinctoria) were more potent than ethyl acetate extract, whereas for all other plants ethyl acetate extracts were more potent. A comparison of sEH inhibition activity of methanolic and ethyl acetate extracts of the plants is represented in fig. 2.
Fig. 2: Comparison of sEH inhibition activity of methanolic and ethyl acetate extracts
Note: The test was conducted in triplicate (n=3) and the data were represented as mean±SEM. *p<0.05 and ***p<0.001 when compared with methanolic extract. Two-way ANOVA followed by Bonferroni post hoc tests, ( ): Methanolic extract and (
): Methanolic extract and ( ): Ethyl acetate extracts
): Ethyl acetate extracts
Out of 50 plant extracts, 31 methanolic extracts and 6 ethyl acetate extracts showed very poor sEH inhibition activity with IC50 values >50 μg/ml. Five plants (Azadirachta indica, P. emblica, Piper nigrum, Psoralea corylifolia and Pungamia glabra) showed sEH inhibition activity with IC50 value >50 μg/ml) in both methanolic and ethyl acetate extracts.
The methanolic extract which showed potent sEH inhibition activity is shown in fig. 3.
Preliminary phytochemical screening indicates that CPME contains components such as alkaloids, carbohydrates, glycosides, proteins, free amino acids and flavonoids, whereas phytosterols, fixed oils, fats, saponins, gums and mucilage were absent. NSME contains components such as alkaloids, carbohydrates, glycosides, proteins, free amino acids and flavonoids, whereas phytosterols, fixed oils, fats, saponins, gums and mucilage were absent. The qualitative phytochemical analysis results of CPME and NSME are shown in Table 2.
| Test components | Test methods | CPME | NSME |
|---|---|---|---|
| Alkaloids | Mayer’s Test | + | + |
| Dragendorff’s test | + | + | |
| Wagner’s test | + | + | |
| Hager’s test | + | + | |
| Carbohydrates | Molisch’s test | + | + |
| Fehling’s test | + | + | |
| Benedict’s test | + | + | |
| Glycosides | Legal’s test | + | + |
| Libermann-Burchard’s test | + | + | |
| Phytosterols | Liebermann- Burchard‘s test | - | - |
| Fixed oils and fats | Spot test | - | - |
| Saponins | Foam test | - | - |
| Phenolic compounds and tannins | Ferric chloride test | + | + |
| Lead acetate test | - | - | |
| Proteins and free amino acids | Millon’s test | + | - |
| Ninhydrin test | + | + | |
| Gums and mucilage | Swelling property | - | - |
| Flavonoids | Shinoda test | + | + |
| Alkaline reagent test | + | + |
Note: (+): Presence and (–): Absence of that particular component
Table 2: Results of Phytochemical Evaluation of CPME and NSME
The quantitative test results (Table 3) indicate that CPME contains 10.6913 (Quercetin Equivalent (QE)/g of total flavonoids, 2.25 μg/ml (equivalent to gallic acid) TPC and 13.24 % of alkaloid content and total saponin content of 1 μg/ml (equivalent to aescin). NSME contains 8.3185 QE/g of total flavonoids, 2.75 μg/ml (equivalent to gallic acid) TPC, 14.20 % of alkaloid content and total saponin content of 0.7 μg/ml (equivalent to aescin).
| Components | Quantities present | |
|---|---|---|
| CPME | NSME | |
| Total flavonoids (equivalent to quercetin) | 10.6913 QE/g | 8.3185 QE/g |
| Total phenolic content (equivalent to gallic acid) | 2.25 µg/ml | 2.75 µg/ml |
| Total saponin content (equivalent to aescin) | 1 µg/ml | 0.7 µg/ml |
| Alkaloid content | 0.1324 | 0.142 |
Table 3: Results of Quantitative Phytochemical Tests of CPME and NSME
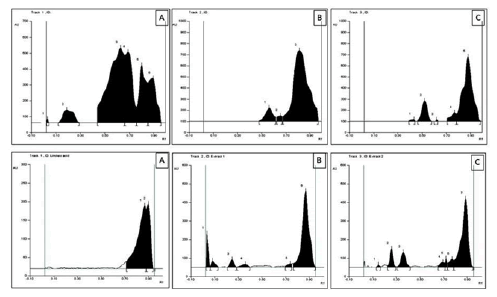
The HPTLC fingerprint studies were carried out for establishing the presence of the biomarker compound linoleic acid. The Rf value of standard linoleic acid was found to be 0.87 at 366 nm. Both CPME and NSME revealed spots having Rf 0.87 at 366 nm. The presence of linoleic acid was confirmed by the overlay spectrum of standard linoleic acid at 366 nm. The linoleic acid, CPME and NSME exhibited blue fluorescence at 254 nm, bright blue fluorescence at 366 nm and no fluorescence at 425 nm. Both extracts were also evaluated for the presence of linoleic acid but could not be confirmed by the overlay spectrum of standard linoleic acid at 254 nm. The HPTLC chromatograms are shown in fig. 4.
COX and LOX are two well-studied enzymatic pathways for synthesizing lipid autacoids endogenously[1-3]. Modulation of these two enzymatic pathways is utilized as a target for treating various pathological conditions associated with inflammatory responses.
Many drugs are available in the market and used extensively for treating inflammation and associated conditions which shows their action through COX and LOX pathways[6]. However, the 3rd enzymatic pathway of endogenous production of lipid autacoids mediated through CYP system is neglected[3]. The EETs produced through the epoxygenase pathways play important role in controlling inflammation as EETs are actively involved in suppressing inflammation through their action on vascular smooth muscles, platelet aggregation, reactive oxygen species generation, nociception and other inflammatory responses[2,3]. Whereas the endogenous enzyme sHE found in a variety of organs, including the liver, heart, spleen, lung and kidney[20], converts EETs to corresponding DHET, a less active compound. Hence, inhibiting sHE spares the highly active EETs and helps for imparting their beneficial anti-inflammatory effects[8,10]. In preclinical models, sEH inhibitors have a substantial anti-inflammatory effect and prevent a variety of pathologic processes, including lung fibrosis, thrombosis and acute respiratory distress syndrome[21]. In addition, sHE inhibition also shows its beneficial effects in many chronic pathological conditions like neurodegeneration and Central Nervous System (CNS) disorders, cardiovascular complications, renal disorders, ulcers, asthma, cancer etc.,[8-12]. Extensive animal hypertension investigations have revealed that EETs vascular, epithelial transport and anti-inflammatory activities lower blood pressure and slow the course of renal and cardiovascular illness[22]. These intriguing findings support the idea that boosting epoxy eicosanoids by sEH inhibitors or EET analogues could be a useful treatment for a variety of chronic conditions. Recent research suggests that aberrant sEH levels may play a role in the development of certain psychiatric illnesses and that sEH inhibitors have antidepressant and antipsychotic action[23].
Hence, in recent decades scientists are focusing on sHE enzyme inhibition as a treatment strategy in many areas. In a similar path in the present study 50 plants with ethno pharmacological importance and previously not reported for sHE enzyme inhibition were evaluated for their action against human sHE activity.
From the studied plants ten methanolic extracts and twenty ethyl acetate extracts have shown potent sHE inhibition potency with IC50 value of less than 10 μg/ ml. These potent plants have been proved beneficial for many chronic pathological conditions including cardiovascular system and CNS complications and cancer. The sHE inhibition potential activities of these plants may be one of the mechanisms through which showed their actions. Hence, the present study data uncover the newer pharmacological target of various plants used in this study. This research work is limited to identifying the plants having sEH inhibition properties in the selected medicinal plants. There is further research scope to screen more and more plants and identify the active constituents responsible for their sEH inhibition activities.
CPME and NSME showed the most potent sEH inhibition activities. The CPME contains components such as alkaloids, carbohydrates, glycosides, proteins, free amino acids and flavonoids, whereas phytosterols, fixed oils, fats, saponins, gums and mucilage were absent. NSME contains components such as alkaloids, carbohydrates, glycosides, proteins, free amino acids and flavonoids, whereas phytosterols, fixed oils, fats, saponins, gums and mucilage were absent.
The quantitative test results indicate that CPME contains 10.6913 QE/g of total flavonoids, 2.25 μg/ ml TPC and 13.24 % of alkaloid content and total saponin content of 1 μg/ml. NSME contains 8.3185 QE/g of total flavonoids, 2.75 μg/ml TPC, 14.20 % of alkaloid content and total saponin content of 0.7 μg/ml. In both CPME and NSME the presence of linoleic acid was confirmed by the overlay spectrum of HPTLC with standard linoleic acid. Hence, the presence of these active compounds may be responsible for their sEH inhibition activities.
In conclusion, the study report suggests that various natural products used in the traditional medicinal system have many promising sEH inhibitors. In the present study, first time we reported sEH inhibition potentials of fifty traditional medicinal plants used in the various system of medicine. From the plants evaluated methanolic extracts of seeds of C. paniculatus and N. sativa has potent sEH inhibition activities. Further research is warranted to identify a greater number of medicinal plants and active principles responsible for sEH inhibition activities.
Conflict of interests:
The authors declared no conflict of interests.
References
- Zhang W, Koerner IP, Noppens R, Grafe M, Tsai HJ, Morisseau C, et al. Soluble epoxide hydrolase: A novel therapeutic target in stroke. J Cereb Blood Flow Metab 2007;27(12):1931-40.
[Crossref] [Google Scholar] [PubMed]
- Piper K, Garelnabi M. Eicosanoids: Atherosclerosis and cardiometabolic health. J Clin Transl Endocrinol 2020;19:100216.
[Crossref] [Google Scholar] [PubMed]
- Panigrahy D, Kaipainen A, Greene ER, Huang S. Cytochrome P450-derived eicosanoids: The neglected pathway in cancer. Cancer Metastasis Rev 2010;29(4):723-35.
[Crossref] [Google Scholar] [PubMed]
- Ribeiro JD, Toro AA, Baracat EC. Antileukotrienes in the treatment of asthma and allergic rhinitis. J Pediatr 2006;82(5):S213-21.
[Crossref] [Google Scholar] [PubMed]
- Cegielska-Perun K, Marczuk E, Bujalska-Zadrozny M. Inhibitors of leukotrienes synthesis: Novel agents and their implementation. Acta Pol Pharm 2016;73(4):843-9.
[Google Scholar] [PubMed]
- Zarriello S, Tuazon JP, Corey S, Schimmel S, Rajani M, Gorsky A, et al. Humble beginnings with big goals: Small molecule soluble epoxide hydrolase inhibitors for treating CNS disorders. Progress in Neurobiol 2019;172:23-39.
[Crossref] [Google Scholar] [PubMed]
- Harris TR, Hammock BD. Soluble epoxide hydrolase: Gene structure, expression and deletion. Gene 2013;526(2):61-74.
[Crossref] [Google Scholar] [PubMed]
- Morisseau C, Hammock BD. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu Rev Pharmacol Toxicol 2013;53:37-58.
[Crossref] [Google Scholar] [PubMed]
- Xu DY, Davis BB, Wang ZH, Zhao SP, Wasti B, Liu ZL, et al. A potent soluble epoxide hydrolase inhibitor, t-AUCB, acts through PPARγ to modulate the function of endothelial progenitor cells from patients with acute myocardial infarction. Int J Cardiol 2013;167(4):1298-304.
[Crossref] [Google Scholar] [PubMed]
- Yang J, Bratt J, Franzi L, Liu JY, Zhang G, Zeki AA, et al. Soluble epoxide hydrolase inhibitor attenuates inflammation and airway hyperresponsiveness in mice. Am J Respir Cell Mol Biol 2015;52(1):46-55.
[Crossref] [Google Scholar] [PubMed]
- Yang L, Mäki-Petäjä K, Cheriyan J, McEniery C, Wilkinson IB. The role of epoxyeicosatrienoic acids in the cardiovascular system. Br J Clin Pharmacol 2015;80(1):28-44.
- Iliff JJ, Jia J, Nelson J, Goyagi T, Klaus J, Alkayed NJ. Epoxyeicosanoid signaling in CNS function and disease. Prostaglandins Other Lipid Mediat 2010;91(3-4):68-84.
[Crossref] [Google Scholar] [PubMed]
- Das Mahapatra A, Choubey R, Datta B. Small molecule soluble epoxide hydrolase inhibitors in multitarget and combination therapies for inflammation and cancer. Molecules 2020;25(23):5488-99.
[Crossref] [Google Scholar] [PubMed]
- Pillarisetti S, Khanna I. Targeting soluble epoxide hydrolase for inflammation and pain-an overview of pharmacology and the inhibitors. Inflamm Allergy Drug Targets 2012;11(2):143-58.
[Crossref] [Google Scholar] [PubMed]
- Thao NP, Luyen BT, Lee JS, Kim JH, Kim YH. Soluble epoxide hydrolase inhibitors of indolinone alkaloids and phenolic derivatives from Cimicifuga dahurica (Turcz.) Maxim. Bioorg Med Chem Lett 2017;27(8):1874-9.
[Crossref] [Google Scholar] [PubMed]
- Kitamura S, Morisseau C, Harris TR, Inceoglu B, Hammock BD. Occurrence of urea-based soluble epoxide hydrolase inhibitors from the plants in the order Brassicales. PloS One 2017;12(5):e0176571.
[Crossref] [Google Scholar] [PubMed]
- Shi DH, Xu C, Guo BX, Wang XT, Chen YX, Tan RX. Inhibition of soluble epoxide hydrolase by extracts derived from inflammation-treating Chinese medicinal herbs. Phytother Res 2008;22(9):1264-8.
[Crossref] [Google Scholar] [PubMed]
- Trease GE, Evans WC. Textbook of Pharmacognosy. 11th London (UK): Balliese, Tindall and Co Publishers; 1989;12:45-50.
- Doss A. Preliminary phytochemical screening of some Indian medicinal plants. Anc Sci Life 2009;29(2):12.
[Google Scholar] [PubMed]
- Liu JY. Inhibition of soluble epoxide hydrolase for renal health. Front Pharmacol 2019;9:1551.
- Hammock BD, Wang W, Gilligan MM, Panigrahy D. Eicosanoids: The overlooked storm in coronavirus disease 2019 (COVID-19)? Am J Pathol 2020;190(9):1782-8.
[Crossref] [Google Scholar] [PubMed]
- Imig JD. Epoxyeicosanoids in hypertension. Physiol Res 2019;68(5):695.
[Crossref] [Google Scholar] [PubMed]
- Ren Q. Soluble epoxide hydrolase inhibitor: A novel potential therapeutic or prophylactic drug for psychiatric disorders. Front Pharmacol 2019;10:420.
[Crossref] [Google Scholar] [PubMed]
- Sahukari R, Punabaka J, Bhasha S, Ganjikunta VS, Kondeti Ramudu S, Kesireddy SR, et al. Phytochemical profile, free radical scavenging and anti-inflammatory properties of Acalyphaindica root extract: Evidence from in vitro and in vivo studies. Molecules 2021;26(20):6251.
[Crossref] [Google Scholar] [PubMed]
- Shamsuddin T, Alam MS, Junaid M, Akter R, Hosen SM, Ferdousy S, et al. Adhatoda vasica (Nees.): A review on its botany, traditional uses phytochemistry, pharmacological activities and toxicity. Mini Rev Med Chem 2021;21(14):1925-64.
[Crossref] [Google Scholar] [PubMed]
- Hossain MD, Urbi Z, Sule A, Rahman KM. Andrographis paniculata (Burm. f.) Wall. ex Nees: A review of ethnobotany, phytochemistry and pharmacology. Sci World J 2014;2014:274-85.
[Crossref] [Google Scholar] [PubMed]
- Gupta SC, Prasad S, Tyagi AK, Kunnumakkara AB, Aggarwal BB. Neem (Azadirachta indica): An Indian traditional panacea with modern molecular basis. Phytomedicine 2017;34:14-20.
[Crossref] [Google Scholar] [PubMed]
- Elangovan A, Ramachandran J, Lakshmanan DK, Ravichandran G, Thilagar S. Ethnomedical, phytochemical and pharmacological insights on an Indian medicinal plant: The balloon vine (Cardiospermum halicacabum). J Ethnopharmacol 2022;291:115143.
[Crossref] [Google Scholar] [PubMed]
- Fatmawati S, Purnomo AS, Bakar MF. Chemical constituents, usage and pharmacological activity of Cassia alata. Heliyon 2020;6(7):e04396.
- Aleem M. Phytochemistry and pharmacology of Celastrus paniculatus: A nootropic drug. J Complement Integr Med 2021.
[Crossref] [Google Scholar] [PubMed]
- Torbati FA, Ramezani M, Dehghan R, Amiri MS, Moghadam AT, Shakour N, et al. Ethnobotany, phytochemistry and pharmacological features of Centella asiatica: A comprehensive review. Adv Exp Med Bio 2021;1308:451-99.
[Crossref] [Google Scholar] [PubMed]
- MK MM, Mishra SH. Comprehensive review of Clerodendrum phlomidis: A traditionally used bitter. Zhong Xi Yi Jie He Xue Bao 2010;8(6):510-24.
[Google Scholar] [PubMed]
- Brailovsky H, Barrera E. A review of the Mexican species of Alloeorhynchus Fieber (Hemiptera: Heteroptera: Nabidae: Prostemmatinae) with description of six new species, new distributional records and key to the species. Zootaxa 2017;4338(2):305-18.
[Crossref] [Google Scholar] [PubMed]
- An S, Jang E, Lee JH. Preclinical evidence of Curcuma longa and its noncurcuminoid constituents against hepatobiliary diseases: A review. Evid Based Complement Altern Med 2020;2020:8761435-51.
[Crossref] [Google Scholar] [PubMed]
- Yirgu A, Chippaux JP. Ethnomedicinal plants used for snakebite treatments in Ethiopia: A comprehensive overview. J Venom Anim Toxins Incl Trop Dis 2019;25.
[Crossref] [Google Scholar] [PubMed]
- Peerzada AM, Ali HH, Naeem M, Latif M, Bukhari AH, Tanveer A. Cyperus rotundus: Traditional uses, phytochemistry and pharmacological activities. J Ethnopharmacol 2015;174:540-60.
[Crossref] [Google Scholar] [PubMed]
- Ashokkumar K, Murugan M, Dhanya MK, Warkentin TD. Botany, traditional uses, phytochemistry and biological activities of cardamom (Elettaria cardamomum (L.) Maton)–A critical review. J Ethnopharmacol 2020;246:112244.
[Crossref] [Google Scholar] [PubMed]
- Dhadde SB, Nagakannan P, Roopesh M, Kumar SA, Thippeswamy BS, Veerapur VP, et al. Effect of embelin against 3-nitropropionic acid-induced Huntington's disease in rats. Biomed Pharmacother 2016;77:52-8.
[Crossref] [Google Scholar] [PubMed]
- Shaikh A, Dhadde SB, Durg S, Veerapur VP, Badami S, Thippeswamy BS, et al. Effect of embelin against lipopolysaccharide-induced sickness behavior in mice. Phytother Res 2016;30(5):815-22.
[Crossref] [Google Scholar] [PubMed]
- Pastorino G, Cornara L, Soares S, Rodrigues F, Oliveira MB. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytother Res 2018;32(12):2323-39.
[Crossref] [Google Scholar] [PubMed]
- de Boer HJ, Cotingting C. Medicinal plants for women's healthcare in southeast Asia: A meta-analysis of their traditional use, chemical constituents and pharmacology. J Ethnopharmacol 2014;151(2):747-67.
[Crossref] [Google Scholar] [PubMed]
- Claimer CS, Mahesh A, Sinilal B, Rao DM, Thangadurai D. Protective effect of Indigofera aspalathoides roots on N-nitrosodiethylamine-induced hepatocarcinogenesis in mice. Indian J Pharm Sci 2012;74(2):157.
[Crossref] [Google Scholar] [PubMed]
- Ronald Steriti ND. Nutritional support for chronic myelogenous and other leukemias: A review of the scientific literature. Alternat Med Rev 2002;7(5):404-9.
[Google Scholar] [PubMed]
- Khan DA, Hassan F, Ullah H, Karim S, Baseer A, Abid MA, et al. Antibacterial activity of Phyllantus emblica, Coriandrum sativum, Culinaris medic, Lawsonia alba and Cucumis sativus. Acta Pol Pharm Drug Res 2013;70(5):855-60.
- Prajapati MS, Patel JB, Modi K, Shah MB. Leucas aspera: A review. Pharmacogn Rev 2010;4(7):85.
[Crossref] [Google Scholar] [PubMed]
- Mahendran G, Rahman LU. Ethnomedicinal, phytochemical and pharmacological updates on Peppermint (Mentha×piperita)—A review. Phytother Res 2020;34(9):2088-139.
[Crossref] [Google Scholar] [PubMed]
- Antony R, Raveendran J, Biju PG. Anti-inflammatory activity of Mollugo cerviana methanolic extract in LPS-induced acute inflammatory RAW 264.7 macrophages. Comb Chem High Throughput Screen 2022;25(10):1661-71.
[Crossref] [Google Scholar] [PubMed]
- Kooti W, Hasanzadeh-Noohi Z, Sharafi-Ahvazi N, Asadi-Samani M, Ashtary-Larky D. Phytochemistry, pharmacology and therapeutic uses of black seed (Nigella sativa). Chin J Nat Med 2016;14(10):732-45.
[Crossref] [Google Scholar] [PubMed]
- Sestili P, Ismail T, Calcabrini C, Guescini M, Catanzaro E, Turrini E, et al. The potential effects of Ocimum basilicum on health: A review of pharmacological and toxicological studies. Expert Opin Drug Metab Toxicol 2018;14(7):679-92.
[Crossref] [Google Scholar] [PubMed]
- Mirunalini S, Krishnaveni M. Therapeutic potential of Phyllanthus emblica (amla): The ayurvedic wonder. J Basic Clin Physiol Pharmacol 2010;21(1):93-105.
[Crossref] [Google Scholar] [PubMed]
- Kaur N, Kaur B, Sirhindi G. Phytochemistry and pharmacology of Phyllanthus niruri: A review. Phytother Res 2017;31(7):980-1004.
[Crossref] [Google Scholar] [PubMed]
- Madhumita M, Guha P, Nag A. Bio-actives of betel leaf (Piper betle): A comprehensive review on extraction, isolation, characterization and biological activity. Phytother Res 2020;34(10):2609-27.
[Crossref] [Google Scholar] [PubMed]
- Salehi B, Zakaria ZA, Gyawali R, Ibrahim SA, Rajkovic J, Shinwari ZK, et al. Piper species: A comprehensive review on their phytochemistry, biological activities and applications. Molecules 2019;24(7):1364-73.
[Crossref] [Google Scholar] [PubMed]
- Sailaja GR, Sriramavaratharajan V, Murugan R, Mallavarapu GR, Chellappan DR. Vasorelaxant property of Plectranthus vettiveroides root essential oil and its possible mechanism. J Ethnopharmacol 2021;274:114048-57.
[Crossref] [Google Scholar] [PubMed]
- Alam F, Khan GN, Asad MH. Psoralea corylifolia L: Ethnobotanical, biological and chemical aspects: A review. Phytother Res 2018;32(4):597-615.
[Crossref] [Google Scholar] [PubMed]
- Moy RL, Levenson C. Sandalwood album oil as a botanical therapeutic in dermatology. J Clin Aesthet Dermatol 2017;10(10):34-9.
- Mili A, Das S, Nandakumar K, Lobo R. A comprehensive review on Sesamum indicum: Botanical, ethnopharmacological, phytochemical and pharmacological aspects. J Ethnopharmacol 2021;281:114503.
[Crossref] [Google Scholar] [PubMed]
- McMurray RL, Ball ME, Tunney MM, Corcionivoschi N, Situ C. Antibacterial activity of four plant extracts extracted from traditional Chinese medicinal plants against Listeria monocytogenes, Escherichia coli and Salmonella enterica enterica serovar Enteritidis. Microorganisms 2020;8(6):962.
[Crossref] [Google Scholar] [PubMed]
- Ganesan K, Sukalingam K, Xu B. Solanum trilobatum ameliorate thioacetamide-induced oxidative stress and hepatic damage in albino rats. Antioxidants 2017;6(3):68.
[Crossref] [Google Scholar] [PubMed]
- Nigam M, Mishra AP, Adhikari-Devkota A, Dirar AI, Hassan MM, Adhikari A, et al. Fruits of Terminalia chebula: A review on traditional uses, bioactive chemical constituents and pharmacological activities. Phytother Res 2020;34(10):2518-33.
[Crossref] [Google Scholar] [PubMed]
- Singh D, Chaudhuri PK. Chemistry and pharmacology of Tinospora cordifolia. Nat Prod Commun 2017;12(2):299-308.
- Kumar SS, Kumar BR, Mohan GK. Hepatoprotective effect of Trichosanthes cucumerina var cucumerina on carbon tetrachloride induced liver damage in rats. J Ethnopharmacol 2009;123(2):347-50.
[Crossref] [Google Scholar] [PubMed]
- Ouzir M, El Bairi K, Amzazi S. Toxicological properties of fenugreek (Trigonella foenumgraecum). Food Chem Toxicol 2016;96:145-54.
[Crossref] [Google Scholar] [PubMed]
- Syahputra RA, Harahap U, Dalimunthe A, Pandapotan M, Satria D. Protective effect of Vernoniaamygdalina Delile against doxorubicin induced cardiotoxicity. Heliyon 2021;7(7):e07434.
[Crossref] [Google Scholar] [PubMed]
- Dogra NK, Kumar S, Kumar D. Vernonia anthelmintica (L.) Willd.: An ethnomedicinal, phytochemical, pharmacological and toxicological review. J Ethnopharmacol 2020;256:112777-89.
[Crossref] [Google Scholar] [PubMed]
- Grover M, Behl T, Virmani T, Bhatia S, Al-Harrasi A, Aleya L. Chrysopogon zizanioides-A review on its pharmacognosy, chemical composition and pharmacological activities. Environ Sci Pollut Res 2021;28(33):44667-92.
[Crossref] [Google Scholar] [PubMed]
- Dar NJ, Hamid A, Ahmad M. Pharmacologic overview of Withania somnifera, the Indian Ginseng. Cell Mol Life Sci 2015;72(23):4445-60.
[Crossref] [Google Scholar] [PubMed]
- Srivastava R. A review on phytochemical, pharmacological and pharmacognostical profile of Wrightia tinctoria: Adulterant of kurchi. Pharmacog Rev 2014;8(15):36-44.
[Crossref] [Google Scholar] [PubMed]
- Mao QQ, Xu XY, Cao SY, Gan RY, Corke H, Beta T, et al. Bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe). Foods 2019;8(6):185.
[Crossref] [Google Scholar] [PubMed]