- *Corresponding Author:
- Shankhadip Nandi
Department of Pharmaceutics, Eminent College of Pharmaceutical Technology, Kolkata, West Bengal 700126, India
E-mail: shankhadipnandi@gmail.com
| Date of Received | 08 August 2022 |
| Date of Revision | 29 June 2023 |
| Date of Acceptance | 25 March 2024 |
| Indian J Pharm Sci 2024;86(2):392-406 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Nanosponge can be considered as an effective carrier among all the novel drug delivery systems based on nanotechnology. It is a newer class of nanoparticulate system having 3-dimensional scaffold structure and nanometric cavity size. Owing to the highly porous nature, nanosponge can offer several benefits like capability to entrap multiple active moieties, loading both hydrophobic and hydrophilic drugs, enhancing the drug solubility followed by promoting the bioavailability of drug molecules, and exhibiting programmable release. These spherical shaped colloidal nanocarriers have the potential to elevate the solubility for poorly soluble drugs through their inclusion and non-inclusion activities. The drugs belonging to Biopharmaceutical Classification System class II and IV are the suitable candidates to be developed as nanosponge formulation for increasing their solubility. Emulsion solvent diffusion method, ultrasound-assisted synthesis, high pressure homogenization etc. are the popular methods for preparation of nanosponge. An extensive variety of additives such as polymers, copolymers, cross-linkers, surfactants are used for releasing the drugs to the target site in a predictable manner through various routes of administration like oral, parenteral, topical. In this review, an overview of nanosponge and its recent advancement in field of drug delivery system along with fu ture perspectives will be discussed.
Keywords
Nanosponge, Biopharmaceutical Classification System class II and IV, emulsion solvent diffusion method, high pressure homogenization
The primary objective of any drug delivery system is to deliver a satisfying amount of drug to the appropriate site of action in the body as well as to achieve and maintain the required plasma concentration of the drug for a certain period of time[1,2]. An ideal drug delivery system should facilitate solubilization of the drug and provide the therapeutic effect to the target site to accomplish the individual need of the patient as well as the disease stage[3]. Targeted drug delivery assists to improve therapeutic efficacy, reduce side effects and get optimized dosing regimen, which is one of the leading trends in the arena of drug delivery[4-6]. Nanosponge (NS) has been emerged as one of the most potential nano-carriers for drug delivery because of their proficiency in controlled and predictable release pattern[7]. NS delivery systems can precisely target the drugs to a specific site of action and have an enormous influence on the medical innovation, particularly improving the demonstrations of existing medication with enabling the new therapies[8-10].
NSs are minute three-dimensional mesh-like scaffold structures with nanosized cavities in which a wide variety of constituents like volatile oil, antineoplastic drugs, proteins and peptides, deoxyribonucleic acid, etc. can be encapsulated[11-13]. The backbone of NS is an elongated polyester thread which is mixed in solution with small molecules termed as crosslinkers that can act similar to the little grappling hooks so that distinct parts of the polymer can be fastened together[14]. Upon reaction between cyclic oligosaccharides known as Cyclodextrins (CDs) and suitable cross-linking reagents, a novel nanomaterial comprising of hyper-cross-linked CDs can be obtained which are popularly known as CD based NSs[15,16]. The polyesters should be biodegradable in nature for which NS can be sufficiently disrupted in the body. The drug loaded in the NS initiates to release when the backbone of NS breaks down[12].
NS illustrates a set of notable advantages in contrast to other available nanoparticulate systems[17]. It can be easily redeveloped by any one of the following actions, such as mild heating, altering the pH or ionic strength, stripping with moderately inert hot gases, washing with eco-compatible diluents[18]. For these remarkable features, researchers have been focused their attention towards the NS for employing them in various applied fields like pharmaceutical, nutraceutical, cosmetics and cosmeceuticals[19-21].
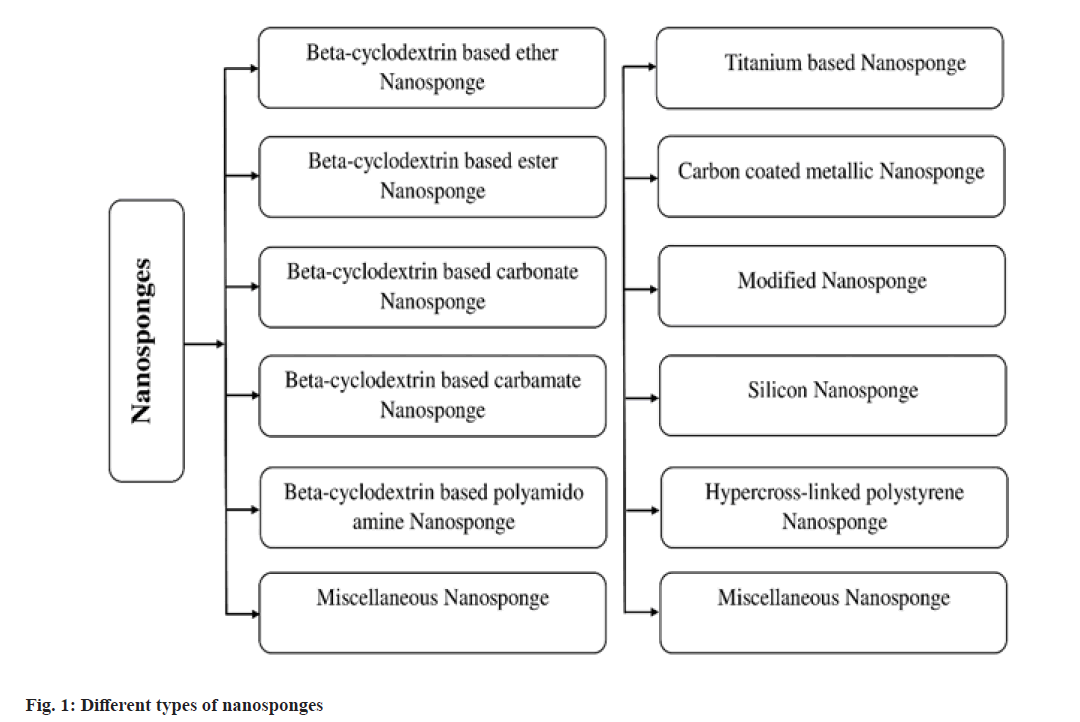
Several categories of NS may be now fabricated depending upon wide range of factors like nature of polymer added, strength of the polymer and the technique of preparation followed. Multiple variations of NS formulation have been already reported in several literatures (fig. 1). Beta CD based NSs are the most explored forms of NS that have been diversely utilized.
NSs are solid, spherical shaped colloidal structure with porous surface. Due to the inclusion and non-inclusion fashion, they have been evidenced to have a very high solubilization capability for the poorly soluble agents especially Biopharmaceutical Classification System (BCS) class II and IV drug candidates[22-24]. NSs can be easily dispersed in a matrix of suitable excipients such as diluents, disintegrants, lubricants, anticaking agents to develop oral dosage forms. They may be also formulated as other dosage forms like parenteral, topical, and inhalation[25,26]. NS loaded tablets and capsules offer a number of crucial benefits like retention of dosage form at the specific site, reduction in estimated dose, reduced toxicity, improved patient compliance followed by prolonged release[24,27]. For parenteral administration, NS can be simply carried with saline, sterile water, or any other aqueous solutions and for topical administration, they can be successfully incorporated into topical hydrogel[28].
Advantages and Limitations of NS
NS may be employed as a therapeutic device for improving the solubility of lipophilic aqueous vehicles, retaining degradable molecules, and developing passages for the transmission of medications at diverse routes of administration including the oral route[29]. It is a proficient carrier for transporting both hydrophilic and lipophilic substances. NS has the ability to increase the solubility of poorly water-soluble substances and achieve improved bioavailability of the drug molecules by altering their pharmacokinetic parameters[30]. It can play a dynamic role in controlled drug delivery at the targeted site due to its lesser particle size with having unique properties like molecular weight ranging from 100-400 dalton and melting point (<250°). NS is stable over a wide range of pH i.e., 1-11 and high temperature up to 130°[31-33]. NSs are non-toxic, non-irritating, nonallergenic, and non-mutagenic[8]. Bacteria cannot penetrate the NS because of their tiny pore size (0.25 μm) and hence they act as self-sterilizer[12]. NS causes enhanced stability, increased elegance and greater flexibility of any formulation[34,35]. It may assist in the process of material transferring i.e., liquid formulation can be converted to powder[36]. NSs are biodegradable[37], free flowing[38], and highly compatible with an extensive variety of substances[39]. They can offer extended release of drugs up to 12 h[40]. NSs are more efficient in the therapy for the breast cancer as compared to conventional medication which often produces several complications[24]. A study carried out by the researchers at Vanderbilt University and Emory University stated that NS drug delivery system may become an promising method for conveying anticancer treatments, facilitating direct injection into tumor sites[8].
The major drawback of NS is their disability to entrap large molecules[30,37]. NSs exist as either in crystalline or para-crystalline form which tends to lack in their drug loading capacity[41]. In para-crystalline form NS can demonstrate altered loading capacities. Dose dumping from the devices may occur occasionally[37].
Chemicals Used for Synthesis of NS
The drug candidates to be encapsulated within NS should have some characteristics. Molecular weight of the drug should range between 100- 400 daltons. Drug molecules must comprise of less than five condensed rings. Melting point of the drug should be less than 250°[42]. Apart from the drug, other required chemicals to synthesize NS are enlisted in Table 1[43-45]. The choice of polymer used in the formulation may impact on the performance of NS. The polymer should be selected depending upon the requisite drug release and category of drug to be entrapped. CD based NSs may be alternatively synthesized through the formation of covalent binds between the CDs and a specific multifunctional reactant known as crosslinker (Table 2)[46]. Cross-linkers are chosen based on the structural features of polymer and the drug to be used in formulation.
| Material | Examples |
|---|---|
| Polymers | Hyper cross‑linked polystyrene, CDs and its derivatives like methyl β‑ cyclodextrin (β‑CD), alkyloxy carbonyl CDs, 2-hydroxy propyl β‑CDs, Eudragit, Ethyl Cellulose, Pluronic F-68 (Poloxamer 188), Pluronic F-127 (Poloxamer 407) |
| Copolymers | Ethyl cellulose, poly (valero lactone‑allylvalero lactone) and poly (valero lactone-allyl valero lactone oxepanedione), polyvinyl alcohol (PVA), hydroxypropyl methylcellulose (HPMC) |
| Cross-linkers | Diphenyl carbonate, di-aryl carbonates, di-isocyanates, acetic acid, pyromellitic anhydride, carbonyl di-imidazole, glutaraldehyde, epichloridrine, carboxylic acid di-anhydrides, 2,2‑bis (acrylamidos), dichloromethane |
| Aprotic solvents | Methanol, ethanol, dimethylacetamide, dimethyl sulphoxide, dimethylformamide |
Table 1: Different Chemicals Used for Synthesis of Ns[43-45]
| Types of CD based NSs | Cross-linkers |
|---|---|
| Carbonate | Carbonyls: diphenyl carbonate (DPC); 1,1′-carbonyl diimidazole (CDI); dimethyl carbonate (DMC) and triphosgene |
| Carbamate | Diisocyanates: 1,6-hexamethylene diisocyanate (HDI); methylene diphenyl diisocyanate (MDI), toluene 2,4-diisocyanate (TDI) and toluene 2,6-diisocyanate |
| Ester | Dianhydrides: pyromellitic dianhydride (PMA); ethylenediaminetetraacetic acid dianhydride (EDTA), Epiclon-B-4400; dialcohol 2-hydroxyethyl disulfide (2-HEDS) can be use together with PMA to introduce disulfide bonds |
| Carboxylic acids: citric acid (CA) and 2,6-naphthalene dicarboxylic acid (NDCA) | |
| Ether | Epoxides: epichlorohydrin; 1,4-butanediol diglycidylether (BDE); E-51 epoxy resin |
| Polyamidoamine | 2,2′-bis(acrylamido)acetic acid and its polyamidoamine derivates (PAA) formed by reaction with amines (such as 2-methylpiperazine) |
| Polyamine | Polyamines: 1,6-hexanediamine (am6), 1,8-octanediamine, 1,12-dodecanediamine (am12) |
Table 2: Cross-Linkers Used in the Preparation of Different Types of Cd Based Ns[46]
Loading of Drug into NS
NS as a drug delivery carrier must be pre-treated in order to achieve an average particle size ranging below 500 nm. The NSs are suspended in water followed by sonication for avoiding the presence of aggregates. The suspension formed is then centrifuged to get colloidal fractions. The supernatant liquid is separated and the sample is dried based on the principle of lyophilization[47].
The aqueous suspension containing NS is formulated and the surplus quantity of drug is diffused throughout the formulation. The formulation is placed under continuous stirring for a predetermined time until the complexation has been occurred[48].
After centrifugation, the portion of undissolved drug can be separated from the complexed drug. The solid crystals of NS are attained by the way of solvent evaporation or applying freeze drying. Structure of the crystals formed caters a very significant role in complexation with drug. A numerous number of research studies showed that the drug loading capacity of NSs is greater in crystalline form than para-crystalline one[47-49]. The drugs that can be complexed within NS formulation are tabulated in the Table 3[50].
| Classification of drugs | Examples |
|---|---|
| Antianxiety drugs | Lorazepam |
| Antibiotics | Azithromycin, erythromycin, cephalexin, ciprofloxacin, ofloxacin, sulfamethoxazole, trimethoprim |
| Anticoagulants | Warfarin |
| Anticonvulsants | Carbamezapine, clonazepam, primidone, felbamate |
| Antihistamines | Terfenadine |
| Antidiabetic and antihyperlipidemic drugs | Atorvastatin, glibenclamide, glipizide, fenofibrate, nateglinide |
| Antineoplastic agents | Camptothecin, etoposide, flutamide, paclitaxel, tamoxifen, docetaxel, exemestane, raloxifene |
| Antifungal | Econazole nitrate, griseofulvin, itraconazole, ketoconazole, lansoprazole, voriconazole |
| Antiepileptic | Phenytoin |
| Antihypertensives | Felodipine, nicardipine, nifedipine, telmisartan |
| Antiarrhythmic agents | Amiodarone hydrochloride |
| Antiretrovirals | Indinavir, ritonavir, saquinavir, nelfinavir |
| Anthelmintics | Albendazole, praziquantel, mebendazole |
| Cardiac drugs | Carvedilol, digoxin, talinolol |
| Immunosuppressants | Cyclosporine, tacrolimus, sirolimus |
Table 3: Examples of Some Drugs Complexed by Using Ns[50]
Methods Available for Fabrication of NS
Solvent method:
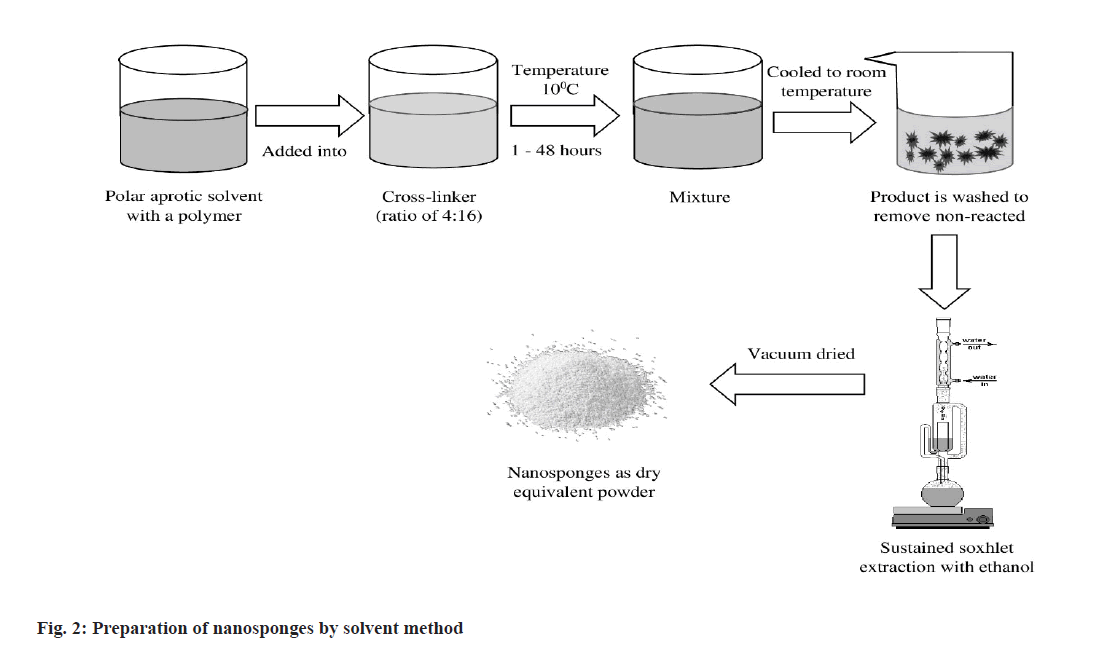
In the solvent method (fig. 2), polymer is mixed with an appropriate solvent, especially in a polar aprotic solvent such as dimethylformamide, dimethyl sulfoxide, etc. The mixture is then gradually added to the excess amount of crosslinker. The molar proportion between cross-linker and polymer should be preferably 4:16. The reaction is generally performed at a specific temperature ranging from 10° to the reflux temperature of the solvent used and continued for about 1-48 h[51].
Carbonyl compounds like dimethyl carbonate, carbonyl di-imidazole are preferred as suitable cross-linkers.
After ending the reaction, the solution is cooled at room temperature and the product is added to excess amount of bi-distilled water. The product is recovered by filtration with the support of vacuum pump and subsequently purified by prolonged soxhlation using ethanol as solvent. The product is then dried under vacuum and grinded in a mechanical mill to obtain desired uniform powder[52].
This method is simple and cost effective which facilitates shorter extraction time with a higher percentage of production yield. But it presents a series of limitations like diminished spherical shape, less homogeneity in size distribution, greater particle size, poor encapsulation efficiency, burst release effect that have pushed further research to find alternative fabrication methods[53].
Emulsion solvent diffusion method:
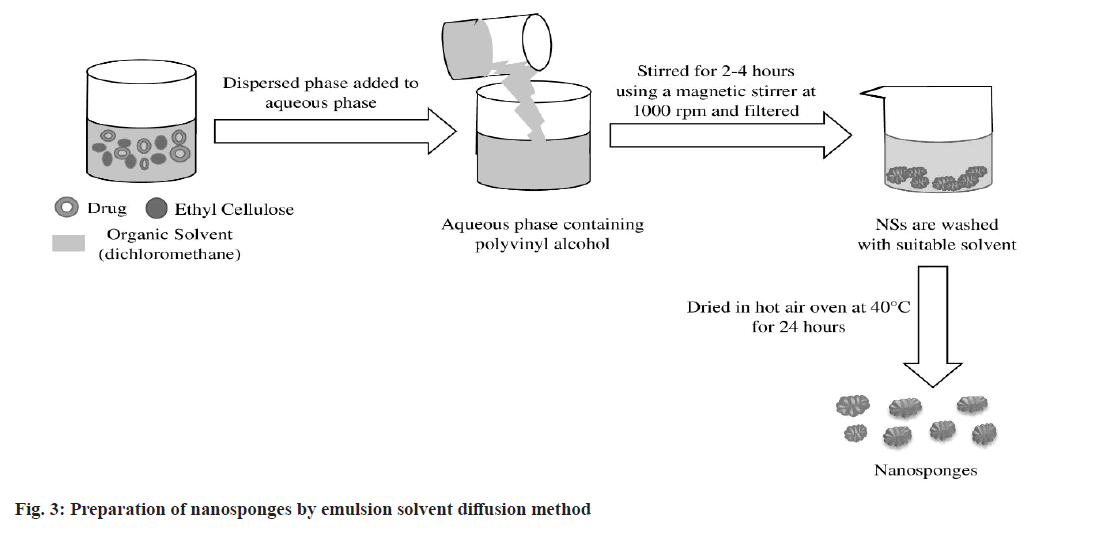
In the emulsion solvent diffusion method (fig. 3), organic dispersed phase and aqueous continuous phase are used to fabricate NS[54]. Different concentrations of ethyl cellulose and polyvinyl alcohol are most commonly used in organic and aqueous phase respectively. A specific amount of drug and ethyl cellulose is dissolved in a suitable organic solvent like dichloromethane. This dispersed phase is then gradually added to aqueous phase consisting polyvinyl alcohol. The reaction mixture is stirred for 2-4 h using a magnetic stirrer maintaining 1000 rpm[55]. The resulting NSs are separated by filtration followed by washing with a suitable solvent. Then they are dried in hot air oven at a temperature 40° for 24 h and stored in a vacuum desiccator[56,57].
This simple and cost effective technique offers several advantages over other fabrication methods like utilization of acceptable organic solvents, high formulation yield, excellent batch-to-batch reproducibility, narrow size distribution. This method is capable to achieve a precise control in particle size that is difficult with other available methods. Major drawback of using this method is leakage of water soluble drug substance into the external phase during emulsification process that may decrease encapsulation efficiency[58-60].
Quasi-emulsion solvent diffusion method:
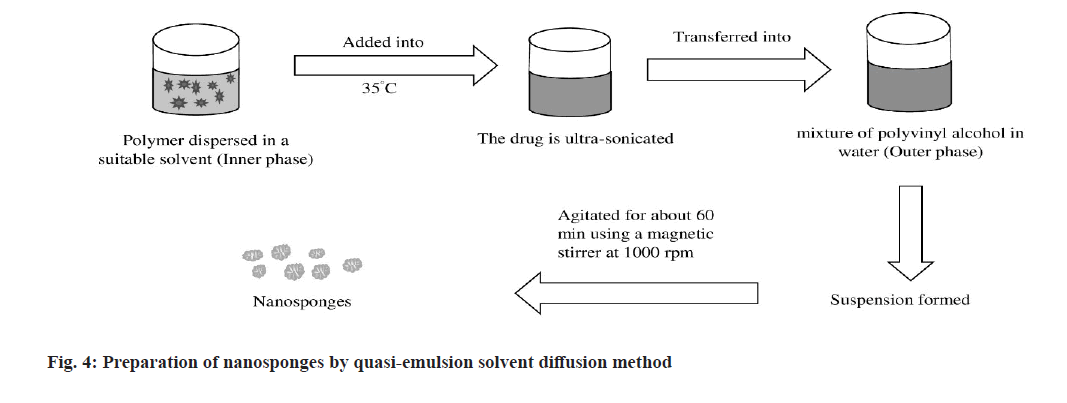
In this solvent diffusion method (fig. 4), polymer is dispersed in a suitable solvent which acts as inner phase[61,62]. The drug is ultra-sonicated and mixed to the solution at a temperature 35°. Then, the inner phase is transferred into the outer phase consisting a mixture of polyvinyl alcohol in water[28]. The formed suspension is then agitated for about 60 min using a magnetic stirrer at 1000 rpm. The resulting NSs are filtered, washed and dried in a hot air oven at 40° for 2 h[63].
High drug loading capacity, low solvent traces, spherical particle shape, etc. are the significant benefits of this method. Size of NS can be easily regulated by controlling the speed of stirring used in this method. Requirement of long time for the reaction of monomers, incompetent to load water soluble drugs-these are the main barriers to follow this method at a large extent[64].
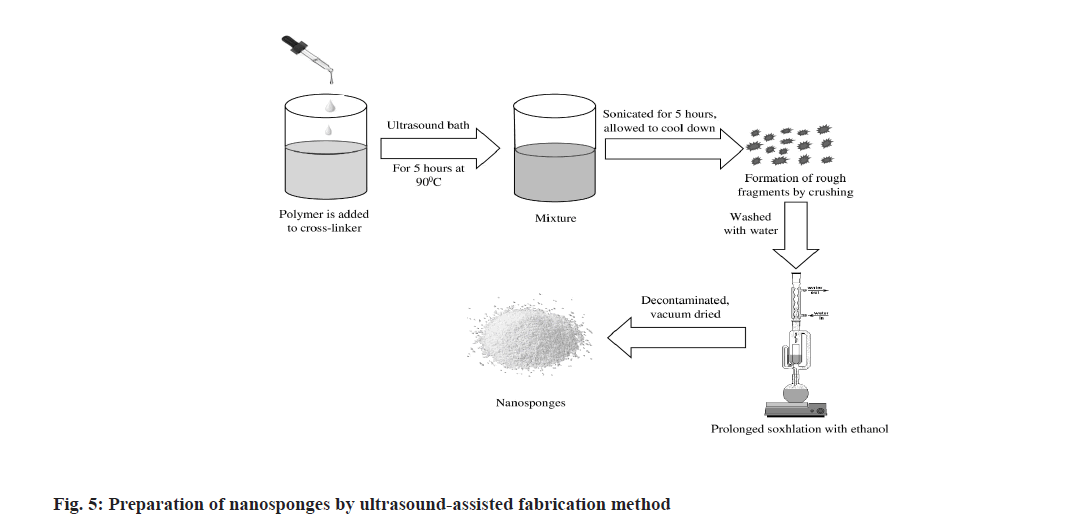
Ultrasound-assisted fabrication method:
In this method (fig. 5), sufficient quantity of polymer is reacted with suitable cross-linkers like diphenyl carbonate or pyromellitic anhydride in a particular molar ratio without using any solvent. The reaction is carried out in a flask which is placed in an ultrasound bath filled with water and heated up to 90°. The mixture is then sonicated for about 5 h and allowed to cool down. The obtained product is smashed into rough fragments. These small fragments are washed with water followed by subsequent prolonged soxhlation with ethanol to remove non-reacting polymers. After decontamination, the resulting NSs are dried under vacuum and stored at a temperature 25° until they are used[65].
A few advantages like no solvent traces, quick reproducible results can be achieved by using the method. Simultaneously, this method produces NS of asymmetrical structure with irregular particle size and it also requires cross-linking agents that may be potentially toxic-these findings make the method limited to use[64].
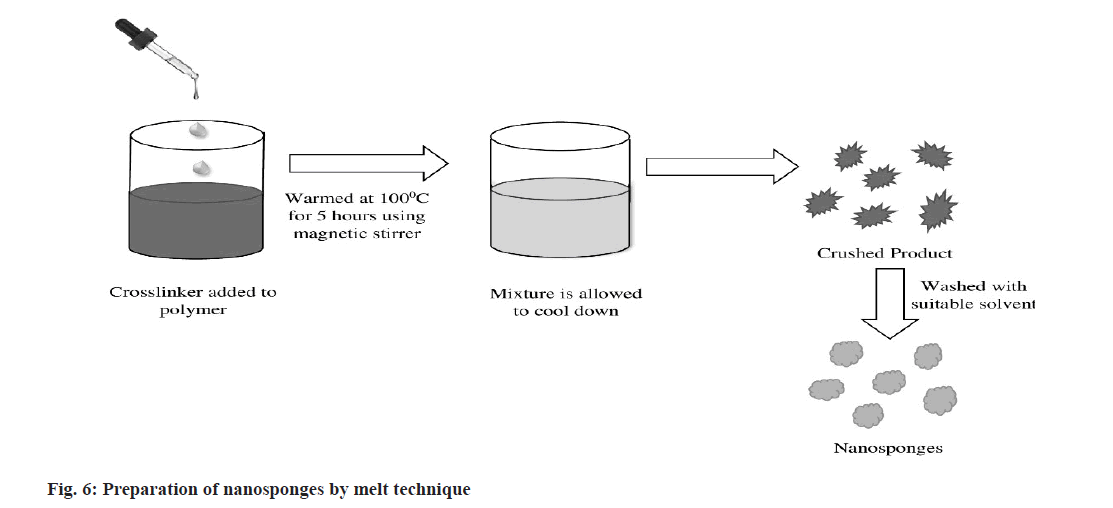
Melt technique:
In this method (fig. 6), CD reacts with suitable cross-linkers such as diaryl carbonates, dimethyl carbonate, isocyanates, diphenyl carbonate, carbonyl diimidazole, carboxylic acid anhydrides. All the ingredients are precisely combined and placed in a 250 ml flask warmed at 100°, and the reaction is carried out for about 5 h using magnetic stirrer. The mixture is then allowed to cool. The product obtained is crushed and washed with a suitable solvent to eliminate unreacted excipients[66-68].
It is a continuous, reproducible, efficient and highly automated process. But, thermal degradation of heat sensitive materials, higher production cost, unavailability of skilled and experienced personnel lead this technique unsuitable for fabrication of NS[68].
Characterization of NS
Solubility studies:
Effectiveness of NS on the solubility of drugs is determined by the plot obtained from phase solubility method explained by Higuchi and Connors[69]. This study demonstrates the degree of complexation as well as pH and bioavailability of the drug[11,70].
Microscopy studies:
Difference in the crystallization state of drug and NS observed under electron microscope specifies the growth of the inclusion moieties. Transmission electron microscope (TEM) and Scanning Electron Microscope (SEM) are commonly employed to visualize the morphology and surface topography of the drug, NS and the complex formed[19,70,71].
Particle size and polydispersity index:
The particle size of NS influences the solubility of drug and also its release. Particle size distribution can be determined by the method Dynamic Light Scattering (DLS) using a software named 90Plus. All the samples are preferably diluted with Milli Q water and every measurement is taken at a fixed angle of 90°. The mean diameter and Polydispersity Index (PDI) can be calculated from the particle size obtained[3,30].
Zeta potential:
Zeta potential is the chief indicator for the stability of the formulation. The value of zeta potential greater than 30 mV specifies the stability of the formulation. Zeta potential determines the surface charge of the NSs prepared. Zeta potential can be defined as the difference in potential between dispersion medium and immobile layer of fluid locked up with dispersed particles. It can be measured by using zeta sizer or adding extra electrode in particle size equipment[72].
Loading efficiency:
The loading or entrapment efficiency of NS can be determined by performing quantitative assessment of drug complexed into the NS using UV spectrophotometer and High-Performance Liquid Chromatography (HPLC). The loading efficiency of NS is calculated by using the equation given below[73].
Loading efficiency=Actual drug content in NS/ Theoretical drug content×100
Thin layer chromatography:
The retention factor (Rf value) of a drug substance diminishes to a significant extent which helps in identifying the complex formation between the drug and NS[74].
Fourier Transform Infrared (FT-IR) analysis:
FT-IR analysis is carried out to check the interaction of chemical bonds between drug and polymer. Samples are scanned in the range from 400-4000 cm-1[54].
Thermodynamical method:
This method determines the changes in drug substances like melting, evaporation, oxidation and decomposition and any polymeric transition before the thermal degradation of NS. These changes in the drug substances indicate the formation of complexes[3].
Porosity:
It is carried out to check the extent of nanochannels and nanocavities formed. Porosity can be assessed by the density of NS. Percent porosity is calculated by using the following equation[9].
Percentage porosity=Bulk volume-True volume/ Bulk volume×100
X-ray diffractiometry analysis:
Powder X-ray diffractiometry is used to discover inclusion complexation in the solid state. Diffraction peaks for a mixture of drug with NS are useful in determining the chemical decomposition and complex formation. The complexation of drug molecule with NS revises in the diffraction patterns and also causes a change in the crystalline nature of the drug. The formation of complexes leads to the sharpening of the present peaks, addition of a few new peaks and shifting of certain peaks[3,75].
Single crystal X-ray structure analysis:
This X-ray structure analysis is carried out to determine the inclusion structure and mode of interaction in detail. The interaction between the molecules can be recognized and the precise geometrical association can be proven[75].
In vitro drug release study:
United States Pharmacopoeia paddle type dissolution apparatus is employed to carry out the in vitro drug release study. The fabricated NS is powdered and placed in the reaction vessel containing 900 ml of phosphate buffer pH 6.8 maintained a temperature 37±0.5°. The apparatus is operated at 75 rpm for 12 h. At specific time intervals samples are withdrawn followed by maintaining sink condition in the vessel, filtered, diluted, and analyzed using uv-visible spectrophotometer[76]. The in vitro drug release study can be also carried out by using dialysis membrane method.
Drug release kinetics:
Drug release kinetics study is performed in order to check the kinetic mechanism of NS involved in the drug release. Zero-order, first-order, higuchi model, korsmeyer–peppas models are most commonly used to check the mechanism of drug release[77].
Applications
Owing to attractive biocompatibility and versatility, NSs have numerous applications in pharmacy. NS can be performed as multifunctional carriers for a wide variety of drugs enlisted in Table 3. They can be also utilized as excipients for manufacturing of different types of dosage forms like pellets, tablets, capsules, granules, solid dispersions, suspensions, etc.[78].
Solubility enhancement:
Swaminathan et al.[19] reported about the enhanced solubility of itraconazole, a BCS Class II drug as NS formulation. Solubility of the drug can be improved by the NSs for more than 27-folds. Addition of copolyvidonum in the NS formulation as a ternary solid dispersion system surpassed the solubility to 55-folds.
NS as carrier of anticancer drugs:
Drugs are dispersed at molecular level when complexed with NS in order to avoid crystallization. Paclitaxel is used as first-line therapy for the treatment of breast and ovary cancers[79], but has extremely low aqueous solubility (30 μg/l). It has been observed that when paclitaxel is complexed as NS it showed high potential to target the site and the organ.
NS as antibiotic delivery:
Satisfactory encapsulation efficiency of doxorobucin in carbonate NS have been observed with loading capacity nearly 20 % w/w. Since doxorubicin is water-soluble or hydrophilic molecule, the NS formulation showed an extendedrelease profile over the time being. Doxorubicin was reported to release very slowly at pH 1.2 (about 1 % after 120 min) and the percentage of drug release increased with pH values. It was observed that at pH 7.4 nearly 29 % of doxorubicin was released after 3 h[17].
Gaseous encapsulation:
NS has been used to develop enclosure complexes with three different gases, viz. carbon dioxide, 1-methylcyclopropene and oxygen. In different biomedical fields, oxygen and carbon dioxide complexion can be useful. NSs were reported as a gas carrier owing to their high porous nature which can release oxygen in extended and continuous manner. α-, β-, or γ-CD, have been found capable to entrap oxygen efficiently for a long period of time. Such variant of NSs have the ability to release oxygen, with or without the effect of ultrasound. By using a combined device comprised of β-CD based NSs with hydrogel, oxygen can be infused via silicone film in a controlled manner. But, the findings from an in vitro release study suggested that the assistance of ultrasound may improve the liberation and entrance of oxygen inside the cell efficiently[61].
NS for topical supply of drugs:
Different formulations intended for external use with using different categories of drug like antibiotics, antifungal, local anesthetics demonstrated the suitability to be complexed as NS[80]. Pushpalatha et al.[81] reported about econazole nitrate NS, an uniformly nano-sized delivery system with impaled orange peel that had been fabricated and characterized by SEM. It can be an effective formulation for the delivery of econazole.
NS as a diagnostic tool:
NS can be broadly used in the manufacturing of an enormous number of diagnostic devices. The different properties exhibited by NS like extended circulation of the blood, biocompatibility, size uniformity for permeability, smooth access towards the target site help to consider them promising tool to be used as a diagnostic agent[82].
NS in cosmetics:
NS has a wide range of usages in the cosmetics industry. NSs have the ability to absorb the unpleasant smell of the human body from sweating and deliver virtuous protection for the cosmetic elements tend to photodegradation. They are proficient to extend the release of volatile constituents gradually and provide a longlasting refreshment when especially used as oral cosmetics[50].
NS in enzyme immobilization:
Ahmed et al.[32] have been reported that Pseudomonas fluorescens lipase adsorbed to a modified NS which is capable to produce highly hydrolytic efficiency.
Drug delivery:
It has been observed that NSs are competent to carry the drug four to five times more effectually to the targeted site as compared to direct injection. They have the potential to be fashioned as several pharmaceutical dosage forms like oral, topical, inhalation, parenteral, etc[17]. The transportation of the formed complex can be proficiently administered through sterile water, saline, or any other suitable fluids for parenteral drug delivery. A multiple number of drugs can be easily encapsulated in the form of hydrogel for topical administration.
Transporter of enzymes, biocatalysts, proteins, vaccines and antibodies:
NS can be efficiently used as suitable carriers for adsorbing enzymes, proteins, antibodies, macromolecules, etc. The utility, performance and temperature range of activity can be expanded when enzymes are applied through NS as transporter. Macromolecules like proteins may be easily relocated to NS either by encapsulating or adsorbing into them. Stability of proteins can be significantly enhanced by forming a complex between NS-based proteins like Bovine Serum Albumin (BSA) and swell-capable polyamidoamine NS[22]. Since NS made from polymeric materials and having large dynamic surface area with several active sites available within them, they are supposed promising constituents to be used as heterogeneous biocatalysts[83].
Other applications:
Yadav et al.[26] reported that different anti-viral drugs like saquinavir, zidovudine, acyclovir, interferon-5-007, etc. are recently engaged in the construction of eudragit based NS formulations. Chhabria et al.[84] discussed about the potential of NS in extracting harmful complexes that are injurious to the blood-flow. NS seems just like a red blood cell in the bloodstream which absorbs toxins by means of targeting and consuming them. NS can be also utilized as a modulated release transporter for water-soluble drugs including proteins and peptides[85].
For the treatment of fungal infections and different skin diseases like ringworm, tinea pityriasis versicolor, vaginal thrush, jock itch, etc. topical therapy of econazole nitrate in the form of solution, cream, lotion, ointment is highly preferred. Kartik et al.[50] reported about the preparation of an emulsion solvent system comprising econazole nitrate NS which can be administered in the form of hydrogel for releasing the drug in a prolonged fashion as topical application.
Recent Advancement in Research Interest Towards NS
A number of drug candidates that have been already designed as different types of NS formulations intended for their diverse applications shown in Table 4[86,87]. In recent times, a multiple number of new patents regarding NS have been filed and approved (Table 5). The patents may be helpful in improving the preparation methods which can make the NS more efficient. The patents have been filed based on the utilization of NS in enzyme immobilization, toxin absorbing properties, biocatalyst studies, antitumoral functions. These granted patents support to accelerate the research interest towards NS that can be emerged as a prospective nanomaterial. A few drugs like alprostadil, dexamethasone, iodine, piroxicam, etc. complexed with NS are now available in market as different dosage forms[37].
| Polymer(s) used | Drug(s) used | Attributes |
|---|---|---|
| α-, βCD:CDI | Oxyresveratrol | Anticancer drug delivery |
| Eudragit | Gliclazide | Antidiabetic drug delivery |
| βCD:PMA | Insulin | Protein delivery |
| Ethyl cellulose | Butenafine hydrochloride | Anti-fungal gel for skin infection |
| HPβCD+βCD:CDI | Artemether+lumefantrine | Drug delivery |
| Ethyl cellulose | Terbinafine hydrochloride | Anti-fungal formulation |
| βCD:DPC | Febuxostat | Oral bioavailability enhancement |
| βCD:DMC | Paracetamol+caffeine+aceclofenac | Solubility enhancement |
| βCD | Piroxicam | Solubility enhancement and improved analgesic activity |
| βCD:CDI | Sulfamethoxazole | Solubility enhancement |
| βCD:CDI | Bortezomib | Anticancer drug delivery |
| Ethyl cellulose | α-mangostin | Antidiabetic therapy |
| βCD:DMC | Curcumin+Caffeine | Topical delivery |
| βCD:PMA | Doxorubicin | Cancer therapy |
| βCD | Griseofulvin | Bioavailability enhancement |
| βCD:PMA, βCD:DPC | Irbesartan | Solubility enhancement |
| Ethyl cellulose | Indomethacin | Sustained release formulation |
| βCD:DPC | Clobetasol propionate | Topical delivery |
| βCD:CDI | Econazole nitrate | Topical delivery |
| βCD:DPC | Sesamol | Photostability enhancement |
| Ethyl cellulose | Isoniazid | Topical delivery |
| βCD:DPC | Limonene essential oil | Solubility enhancement and volatility reduction |
| Pluronic F-68 | Voriconazole | Treatment of chronic fungal infection |
| βCD:CDI | Flutamide | Anticancer drug delivery |
| Ethyl cellulose | Lansoprazole | Treatment of ulcer |
| βCD:DPC | Thyme essential oil | Solubility enhancement and volatility reduction |
Table 4: Pharmaceutical Applications of Ns Formulations[13,81,82]
| Patent No. | Title | Applicant |
|---|---|---|
| W02006002814A1 | Ultrasound-assisted synthesis of cyclodextrin-based nanosponges | Francesco Trotta, Wander Tumiatti, Orfeo Zerbinati, Carlo Roggero, Roberto Vallero |
| W02012147069A1 | Method of preparing dextrin nanosponges | Universita DegliStudi Di Torino, Sea Marconi, Technologies Di |
| WO2007095454A2 | Carbon-encased metal nanoparticles and sponges, methods of synthesis, and methods of use | Kun Lian, Qinglin Wu |
| WO2012147069A1 | Method for preparing dextrin nanosponges | Francesco Trotta, Pravin Shende, Miriam Biasizzo |
| WO2009138998A3 | A template free and polymer free metal nanosponge and a process thereof | Eswaramoorthy Muthusamy, Saikrishana Katla |
| W02009149883A1 | Cyclodextrin nanosponges as a carrier for biocatalysts, and in the delivery and release of enzymes, proteins, vaccines and antibodies | Gianfranco Gilardi, Francesco Trotta, Roberto Cavalli, Paolo Ferruti, Elisabetta Ranucci, Giovanna Di Nardo, Carlo Mario Roggero, Vander Tumiatti |
| US9574136B2 | Nanoparticles, nanosponges, methods of synthesis, and methods of use | Kun Lian |
| US8828485B2 | Carbon-encased metal nanoparticles and sponges as wood/plant preservatives or strengthening fillers | Kun Lian, Qinglin Wu |
| US20170152439A1 | Nanoparticles, nanosponges, methods of synthesis, and methods of use | Kun Lian |
| CA2692493A1 | Cyclodextrin-based nanosponges as a vehicle for antitumoral drugs | Sea Marconi, Technologies Di Vander Tumiatti, S.A.S, Francessco Trotta, Vander Tumiatti, Roberta Cavalli, Carlo Mario Roggero, Barbar Mognetti, Giovanni, Nicolao Berta |
| ITMI20071321A1 | Nanosponges based on cyclodextrins as a vehicle for anticancer drugs | Giovanni Nicolao Berta, Roberta Cavalli, Barbara Mognetti, Carlo Maria Roggero, Francesco Trotta, Vander Tumiatti |
Table 5: Recently Granted Patents on Ns[50]
Future Perspectivies and Challenges
The unique nature exhibited by NS in terms of synthesis, gradation of crosslinking upon drug release, particle size, crystallinity, porosity will support them to play a dominant role in the medical and pharmaceutical field. But traditional protocol used for the synthesis of NS with the presence of toxic organic vehicles, homogeneous catalysis methods, formation of chiral stereogenic metal complexes make them unsustainable. Ultrasound assisted synthesis is considered as the most effective method of preparation due to its traditional approach, but the novel methods like solvent evaporation and bubble electrospinning are also being updated and developed. Preparation methods of NS are simple but the presence of residual liquid is treated as major flaw in the chemical process in the obtained product which may exhibit noxious effects[82]. Increased rate of yields, reproducibility, cost-effective fabrication and bulk production in a short period of time bring NS as a trending option for drug delivery. It is strongly believed that NSs have an auspicious and sustainable perspective not only in pharmaceutical and medical field but also in a wide range of unrevealed field which may be disclosed in near future.
Conclusion
NSs have the ability to incorporate active fragments to a great extent. They may be fabricated by simple synthetic procedure, even though a onestep synthetic direction, without use of any toxic solvent or involvement of any instruments. At the same time, production of NS is cost-effective as compared to few nanocarriers like niosomes, ethosomes, liposomes, solid lipid nanoparticles, etc. Although sizes and shape of nanoparticulate systems are important factors as the in vivo fate after administration depends on it. In a nutshell, NSs provide a flexible and promising drug carrier with ability of site-specific delivery. They can entrap an extensive series of medications especially for oral and topical delivery of drugs. They are also beneficial of encapsulating and transporting both hydrophilic and lipophilic drugs. NSs have broader range of applications in several trails like solubility enrichment, carrier as anticancer and antiviral drugs, entrapment of oxygen, designing as cosmetics, diagnostic devices, poisoning adsorbents, and many more. Multiple assessments are being conducted to determine the structural and chemical veracity of the product. They will be beneficial for the several fields of health care system in the recent future, and their scope of application will be also enlightened as the research interest regarding this nanomaterial expands.
Acknowledgements:
S. Nandi carried out the literature survey, analyzed the data and drafted the manuscript. P. Biswas designed and illustrated the figures. S. Nandi and P. Biswas revised the manuscript as per comments from the reviewers. The authors have read and agreed to the submitted version of the manuscript.
Conflict of interest
The authors declare no competing interests.
References
- Subramanian S, Singireddy A, Krishnamoorthy K, Rajappan M. Nanosponges: A novel class of drug delivery system-review. J Pharm Pharm Sci 2012;15(1):103-11.
[Crossref] [Google Scholar] [PubMed]
- Francis DJE, Yusuf F. Development and evaluation of nanosponges loaded extended release tablets of lansoprazole. Uni J Pharm Res 2019;4(1):24-8.
- Shringirishi M, Prajapati SK, Mahor A, Alok S, Yadav P, Verma A. Nanosponges: A potential nanocarrier for novel drug delivery-a review. Asian Pac J Trop Dis 2014;4(S2):S519-26.
- Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MP, Acosta-Torres LS, et al. Nano based drug delivery systems: Recent developments and future prospects. J Nanobiotechnology 2018;16(1):71.
[Crossref] [Google Scholar] [PubMed]
- Vyas SP, Khar RK. Novel carrier systems. Molecular basis of targeted drug delivery. Targeted and Controlled Drug Delivery 2012;38-40.
- Shivani S, Poladi KK. Nanosponges-novel emerging drug delivery system: A review. Int J Pharm Sci Res 2015;6(2):529-40.
- Rybniker J, Vocat A, Sala C, Busso P, Pojer F, Benjak A, et al. Lansoprazole is an antituberculous prodrug targeting cytochrome bc1. Nat Commun 2015;6(1):7659.
[Crossref] [Google Scholar] [PubMed]
- Jilsha G, Viswanad V. Nanosponges: A novel approach of drug delivery system. Int J Pharm Sci Rev Res 2013;19(2):119-23.
- Penjuri SCB, Ravouru N, Damineni S, Sailakshmi BNS, Poreddy SR. Formulation and evaluation of lansoprazole loaded nanosponges. Turk J Pharm Sci 2016;13(3):304-10.
- Cheng Y, Morshed RA, Auffinger B, Tobias AL, Lesniak MS. Multifunctional nanoparticles for brain tumor imaging and therapy. Adv Drug Deliv Rev 2014;66:42-57.
[Crossref] [Google Scholar] [PubMed]
- Trotta F, Zanetti M, Cavalli R. Cyclodextrin-based nanosponges as drug carriers. Beilstein J Org Chem 2012;8(1):2091-9.
[Crossref] [Google Scholar] [PubMed]
- Bhowmik H, Venkatesh DN, Kuila A, Kumar KH. Nanosponges: A review. Int J App Pharm 2018;10(4):1-5.
[Crossref]
- Moin A, Roohi NKF, Rizvi SMD, Ashraf SA, Siddiqui AJ, Patel M, et al. Design and formulation of polymeric nanosponge tablets with enhanced solubility for combination therapy. RSC Adv 2020;10(57):34869-84.
[Crossref] [Google Scholar] [PubMed]
- Shinde G, Kesarla R, Bhatt D, Bangale G, Umalkar D, Virag G. Current status of colloidal system (nano range). Int J Drug Formul Res 2011;2(1):39-54.
- Trotta F, Cavalli R. Characterization and applications of new hyper cross-linked cyclodextrins. Composite Interfaces 2009;16(1):39-48.
- Hoti G, Caldera F, Cecone C, Pedrazzo AR, Anceschi A, Appleton SL, et al. Effect of the cross-linking density on the swelling and rheological behavior of ester-bridged β-cyclodextrin nanosponges. Materials 2021;14(3):478.
[Crossref] [Google Scholar] [PubMed]
- Cavalli R, Trotta F, Tumiatti W. Cyclodextrin-based nanosponges for drug delivery. J Incl Phenom Macrocycl Chem 2006;56(2):209-13.
- Nardo GD, Roggero C, Campolongo S, Valetti F, Trotta F, Gilardi G. Catalytic properties of catechol 1,2-dioxygenase from Acinetobacter radioresistens S13 immobilized on nanosponges. Dalton Trans 2009;7(33):6507-12.
[Crossref] [Google Scholar] [PubMed]
- Swaminathan S, Vavia PR, Trotta F, Torne S. Formulation of betacyclodextrin based nanosponges of itraconazole. J Incl Phenom Macrocycl Chem 2007;57:89-94.
- Torne SJ, Ansari KA, Vavia PR, Trotta F, Cavalli R. Enhanced oral paclitaxel bioavailability after administration of paclitaxel-loaded nanosponges. Drug Deliv 2010;17(6):419-25.
[Crossref] [Google Scholar] [PubMed]
- Liang L, Liu DP, Liang CC. Optimizing the delivery systems of chimeric RNA. DNA oligonucleotides. Eur J Biochem 2002;269(23):5753-8.
[Crossref] [Google Scholar] [PubMed]
- Panda S, Vijayalakshmi Sv, Pattnaik S, Swain RP. Nanosponges: A novel carrier for targeted drug delivery. Int J PharmTech Res 2015;8(7):213-24.
- Swaminathan S, Vavia PR, Trotta F, Cavalli R, Tumbiolo S, Bertinetti L, et al. Structural evidence of differential forms of nanosponges of beta-cyclodextrin and its effect on solubilization of a model drug. J Incl Phenom Macrocycl Chem 2013;76:201-11.
- Alongi J, Poskovic M, Frache A, Trotta F. Role of β-cyclodextrin nanosponges in polypropylene photooxidation. Carbohyd Polym 2011;86(1):127-35.
- Poornima, Priya S. Gastroretentive floating tablets enclosing nanosponge loaded with lafutidine for gastric ulcer: Formulation and evaluation. Ind J Pharm Edu Res 2021;55:s100-11.
- Yadav G, Panchory H. Nanosponges: A boon to the targeted drug delivery system. J Drug Deliv Ther 2013;3(4):151-55.
- Lala R, Thorat A, Gargote C. Current trends in β-cyclodextrin based drug delivery systems. Int J Res Ayur Pharm 2011;2(5):1520-26.
- Sharma R, Walker RB, Pathak K. Evaluation of kinetics and mechanism of drug release from econazole nitrate nanosponge loaded carbapol hydrogel. Ind J Pham Edu Res 2011;45(1):25-31. [Crossref]
- Pushpalatha R, Selvamuthukumar S, Kilimozhi D. Cross-linked, cyclodextrin-based nanosponges for curcumin delivery-physicochemical characterization, drug release, stability and cytotoxicity. J Drug Deliv Sci Technol 2018;45:45-53.
- Sadhasivam J, Sugumaran A, Narayanaswamy D. Nano sponges: A potential drug delivery approach. Research J Pharm and Tech 2020;13(7):3442-48.
- Thakre AR, Gholse YN, Kasliwal RH. Nanosponges: A novel approach of drug delivery system. J Med Pharm Allied Sci 2016;5(6):78-92.
- Ahmed RZ, Patil G, Zaheer Z. Nanosponges-a completely new nano-horizon: pharmaceutical applications and recent advances. Drug Dev Ind Pharm 2013;39(9):1263-72.
[Crossref] [Google Scholar] [PubMed]
- Aritomi H, Yamasaki Y, Yamada K, Honda H, Koshi M. Development of sustained release formulation of chlorpheniramine maleate using powder coated microsponges prepared by dry impact blending method. J Pharm Sci Technol 1996;56(1):49-56.
- Patel EK, Oswal RJ. Nanosponge and micro sponges: A novel drug delivery system. Int J Res Pharm Chem 2012;2(2):237-44.
- Targe BM, Patil MP, Jahagirdar AC, Khandekar BD. Nanosponges-an emerging drug delivery system. Int J of Insti Pharm Life Sci 2015;5(6):160-74.
- Nitish, Jeganath S, Fathelrahman K, Abdelmagid K. A review on nanosponges: A promising novel drug delivery system. Research J Pharm and Tech 2021;14(1):501-5.
- Sing D, Soni GC, Prajapati SK. Recent advances in nanosponges as drug delivery system: A review article. Eur J Pharm Med Res 2016;3(10):364-71.
- Narender BR, Sridhar PR. Formulation and evaluation of anticancer drug (tamoxifen) loaded nanosponges. Am J Pharm Health Res 2019;7(12):39-57.
- Mamtha DP, Viresh KC, Shabaraya AR. Nanosponges: An overview about the novel class of drug delivery system. World J Pharm Pharma Sci 2021;10(2):1014-27.
- Vishwakarma A, Nikam P, Mogal R, Talele S. Nanosponges: A benification for novel drug delivery. Int J PharmTech Res 2014;6(1):11-20.
- Dubey P, Sharma HK, Shah S, Tyagi CK, Chandekar AR, Jadon RS. Formulations and evaluation of Cyclodextrin complexed Ceadroxil loaded nanosponges. Int J Drug Deliv 2017;9(3):84-100.
- Vyas A, Saraf S, Saraf S. Cyclodextrin based novel drug delivery systems. J Incl Phenom Macrocycl Chem 2008;62:23-42.
- Tiwari H, Mahor A, Dixit ND, Kushwaha M. A review on nanosponges. World J Pharm Pharm Sci 2014;3:219-33.
- Kumar S, Rao R. Analytical tools for cyclodextrin nanosponges in pharmaceutical field: A review. J Inclusion Phenomena Macrocyclic Chem 2019;94:11-30.
- Darandale SS, Vavia PR. Cyclodextrin-based nanosponges of curcumin: Formulation and physicochemical characterization. J Incl Phenom Macrocycl Chem 2013;75:315-22.
- Utzeri G, Matias PM, Murtinho D, Valente AJ. Cyclodextrin-based nanosponges: Overview and opportunities. Front chEm 2022;10:859406.
- Setijadi E, Tao L, Liu J, Jia Z, Boyer C, Davis TP. Biodegradable star polymers functionalized with β-cyclodextrin inclusion complexes. Biomacromolecules 2009;10(9):2699-707.
[Crossref] [Google Scholar] [PubMed]
- Pawar AY. Nanosponges: A novel drug delivery system. Asian J Pharm 2016;10(4):S456-63.
[Crossref] [Google Scholar] [PubMed]
- Ahire PS, Bhambere DS, Patil MP, Kshirsagar SJ. Recent advances in nanosponges as a drug delivery system. Int J Pharm Sci Nanotech 2013;6(1):1934-44.
- Tiwari K, Bhattacharya S. The ascension of nanosponges as a drug delivery carrier: Preparation, characterization, and applications. J Mater Sci Mater Med 2022;33(3):28.
[Crossref] [Google Scholar] [PubMed]
- Jagtap SR, Bhusnure OG, Mujewar IN, Gholve SB, Panchabai VB. Nanosponges: A novel trend for targeted drug delivery. J Drug Deliv Ther 2019;9(3-s):931-8.
- Yurtdaş G, Demirel M, Genç L. Inclusion complexes of fluconazole with β-cyclodextrin: Physicochemical characterization and in vitro evaluation of its formulation. J Incl Phenom Macrocycl Chem 2011;70:429-35.
- Pavanetto F, Conti B, Genta I, Giunchedi P. Solvent evaporation, solvent extraction and spray drying for polylactide microsphere preparation. Int J Pharm 1992;84(2):151-9.
- Richhariya N, Prajapati SK, Sharma UK. Nanosponges: An innovative drug delivery system. World J Pharm Res 2015;4(7):1751-3.
- Patel B, Bagade O, Ramteke K, Patel R, Awsarkar V. An assessment on preparations, characterization, and poles apart appliances of nanosponge. Int J Pharmtech Res 2014;6(7):2092-101.
- Almutairy BK, Alshetaili A, Alali AS, Ahmed MM, Anwer MK, Aboudzadeh MA. Design of olmesartan medoxomil-loaded nanosponges for hypertension and lung cancer treatments. Polymers 2021;13(14):2272.
[Crossref] [Google Scholar] [PubMed]
- Krishna AV, Gowda VD, Karki R. Formulation and evaluation of nanosponges loaded bifonazole for fungal infection. Anti-Infective Agents 2021;19(1):64-75.
- Palamoor M, Jablonski MM. Comparative study on diffusion and evaporation emulsion methods used to load hydrophilic drugs in poly (ortho ester) nanoparticle emulsions. Powder Technol 2014;253:53-62.
- Chaudhary SA, Patel DM, Patel JK, Patel DH. Solvent emulsification evaporation and solvent emulsification diffusion techniques for nanoparticles. In: Patel JK, Pathak YV, editors. Emerging Technologies for Nanoparticle Manufacturing. 1st ed. Switzerland: Springer Cham; 2021. p. 287-300.
- Pineda-Reyes AM, Delgado MH, de la Luz Zambrano-Zaragoza M, Leyva-Gómez G, Mendoza-Muñoz N, Quintanar-Guerrero D. Implementation of the emulsification-diffusion method by solvent displacement for polystyrene nanoparticles prepared from recycled material. RSC Adv 2021;11(4):2226-34.
- Jadhav PA, Jadhav SA. Review on: Nanosize delivery system. World J Pharm Pharm Sci 2017;6(9):433-44.
- Mukherjee B. Nanosize drug delivery system. Curr Pharm Biotechnol 2013;14:1221.
- Embil K, Nacht S. The microsponge® delivery system (MDS): A topical delivery system with reduced irritancy incorporating multiple triggering mechanisms for the release of actives. J Microencapsul 1996;13(5):575-88.
[Crossref] [Google Scholar] [PubMed]
- Srivastava R, Pathak K. Microsponges: A futuristic approach for oral drug delivery. Exp Opin Drug Deliv 2012;9(7):863-78.
[Crossref] [Google Scholar] [PubMed]
- Cavalli R, Akhter AK, Bisazza A, Giustetto P, Trotta F, Vavia P. Nanosponge formulations as oxygen delivery systems. Int J Pharm 2010;402(1-2):254-7.
[Crossref] [Google Scholar] [PubMed]
- Jyoti P, Tulsi B, Popin K, Chetna B. An innovative advancement for targeted drug delivery: Nanosponges. Indo Global J Pharm Sci 2016;6(2):59-64.
- Simranjot K, Sandeep K. Nanosponges: Present aspects and future challenges. Indo Am J Pharm Sci 2018;5(9):9390-8.
- Ajinkya K, Prakash K, VISHAL P. Scaffold based drug delivery system: A special emphasis on nanosponges. Int J Pharm Drug Anal 2015:98-104.
- Waghmare SG, Nikhade RR, Satish D, Kosalge B. Nanosponges: A novel approach for controlled release drug delivery system. Int J Pharm Pharm Res 2017;9(3):101-1.
- Kfoury M, Landy D, Fourmentin S. Characterization of cyclodextrin/volatile inclusion complexes: A review. Molecules 2018;23(5):1204.
[Crossref] [Google Scholar] [PubMed]
- Tambe RS, Battase PW, Arane PM, Palve SA, Talele SG, Chaudhari G. Review on nanosponges: As a targeted drug delivery system. Am J Pharmtech Res 2015;5(1):215-24.
- Minelli R, Cavalli R, Fantozzi R, Dianzani C, Pettazzoni P, Ellis L, et al. Antitumor activity of nanosponge-encapsulated Camptotechin in human prostate tumors. Cancer Res 2011;71(8):4431.
- Aggarwal G, Nagpal M, Kaur G. Development and comparison of nanosponge and niosome based gel for the topical delivery of tazarotene. Pharm Nanotechnol 2016;4(3):213-28.
[Crossref] [Google Scholar] [PubMed]
- Moura FC, Lago RM. Catalytic growth of carbon nanotubes and nanofibers on vermiculite to produce floatable hydrophobic “nanosponges” for oil spill remediation. Appl Catalysis B Environ 2009;90(3-4):436-40.
- Tayade P, Vavia P. Inclusion complexes of Ketoprofen with-cyclodextrins: Oral pharmacokinetics of Ketoprofen in human. Indian J Pharm Sci 2006;68(2).
- Nandi S, Banerjee A, Reza KH. Formulation and evaluation of enteric coated elementary osmotic tablets of aceclofenac. Turk J Pharm Sci 2021;18(4):498.
[Google Scholar] [PubMed]
- Srinivas P, Jahnavi Reddy A. Formulation and evaluation of isoniazid loaded nanosponges for topical delivery. Pharm Nanotechnol 2015;3(1):68-76.
- Moya-Ortega MD, Alvarez-Lorenzo C, Concheiro A, Loftsson T. Cyclodextrin-based nanogels for pharmaceutical and biomedical applications. Int J Pharm 2012;428(1-2):152-63.
[Crossref] [Google Scholar] [PubMed]
- Malingre MM, Beijnen JH, Schellens JH. Oral delivery of taxanes. Invest New Drugs 2001;19(2):155-62.
[Crossref] [Google Scholar] [PubMed]
- Ananya KV, Preethi S, Patil AB, Gowda DV. Recent review on Nano sponge. Int J Res Pharm Sci 2020;11(1):1085-96.
- Pushpalatha R, Selvamuthukumar S, Kilimozhi D. Cyclodextrin nanosponge based hydrogel for the transdermal co-delivery of curcumin and resveratrol: Development, optimization, in vitro and ex vivo evaluation. J Drug Deliv Sci Technol 2019;52:55-64.
- Gidwani B, Vyas A. A comprehensive review on cyclodextrin-based carriers for delivery of chemotherapeutic cytotoxic anticancer drugs. Biomed Res Int 2015;2015:198268.
[Crossref] [Google Scholar] [PubMed]
- Sabzi NE, Kiasat AR. β-Cyclodextrin based nanosponge as a biodegradable porous three-dimensional nanocatalyst in the one-pot synthesis of n-containing organic scaffolds. Catalysis Lett 2018;148:2654-64.
- Chhabria V, Beeton S. Development of nanosponges from erythrocyte ghosts for removal of streptolysin-O from mammalian blood. Nanomedicine 2016;11(21):2797-807.
[Crossref] [Google Scholar] [PubMed]
- Kumar S, Dalal P, Rao R. Cyclodextrin nanosponges: A promising approach for modulating drug delivery. Colloid Sci Pharm Nanotechnol 2020;79.
- Appleton SL, Tannous M, Argenziano M, Muntoni E, Rosa AC, Rossi D, et al. Nanosponges as protein delivery systems: Insulin, a case study. Int J Pharm 2020;590:119888.
[Crossref] [Google Scholar] [PubMed]
- Amin OM, Ammar A, Eladawy SA. Febuxostat loaded β-cyclodextrin based nanosponge tablet: An in vitro and in vivo evaluation. J Pharm Invest 2020;50:399-411.