- *Corresponding Author:
- P. S. Sellamuthu
Department of Food Process Engineering, Postharvest Research Lab, SRM Institute of Science and Technology, Chennai, Tamil Nadu 603203, India
E-mail: periyar.india@gmail.com
| Date of Received | 10 February 2022 |
| Date of Revision | 08 May 2023 |
| Date of Acceptance | 19 February 2024 |
| Indian J Pharm Sci 2024;86(1):9-18 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Food borne pathogens (bacteria, viruses, parasites, fungi or their mycotoxins) are the major cause of substantial number of diseases with an important impact on human well-being and economy. Contamination or spoilage of food by pathogens is linked with a diverse range of outbreaks of food-borne diseases. World Health Organization defined food-borne disease as an infectious disease which is caused by contaminated food or water. However, antibiotics are vital tools used in the healthcare sector to antagonize food-borne pathogens. The frequent usage of antibiotics has resulted in antimicrobial resistance which is causing major threats throughout the world. Over the past years, there has been an increasing interest in the use of probiotic bacteria as alternatives to antibiotics. Generally, the use of probiotics improves gastrointestinal well-being has been suggested for several years however many critical issues arise in the use of probiotics. Therefore, the notion of paraprobiotics and postbiotics are comparatively novel concepts which do not fit in terms of probiotics. Paraprobiotics and postbiotics are produced from probiotic organisms which provide beneficial impacts on food commodities and human health. This review will provide insight into probiotics, paraprobiotics and postbiotics against food-borne pathogens; and their probiotic challenges.
Keywords
Postbiotics, paraprobiotics, beneficial microbes, health benefits, pathogens
The link between the food consumption and human diseases was primarily reported in 460 BC. Food-borne illness or diseases are major public health issue affecting human health and food safety around the world[1]. The main cause for food-borne illness is consumption foodstuff or animal product contaminated with pathogens (bacteria, viruses, parasites, fungi or their mycotoxins)[2,3]. According to the 2018 World Bank report Food Borne Diseases (FBD) affect nation economy in low and mild incoming countries. Globalization, climate change, inadequate food safety regulation and poor sanitation and food handling practices are the common cause for outbreak of FBD. More than 200 types of food borne illness have been recognized and enormous numbers of people are affected by FBD. FBD are a rising worldwide concern owing to their growing mortality and morbidity. In 2010, FBD Burden Epidemiology Reference (FERG) was established by World Health Organization (WHO) to measure the food borne illness globally[4]. In accordance with the various reports, FBD is a major problem across worldwide which causes death and illness in million people annually[5]. To diminish the food borne pathogens, various control methods should be adopted in order to decrease the occurrence of FBD and food spoilage through pathogens[6,7]. The uses of biological, chemical and physical techniques are generally employed alone or together with other methods to preserve the food so as to reduce food spoilage by microorganisms[8,9]. Among these methods, chemical preservation is the most common approach for food preservation to regulate pH, to serve as antioxidant and antimicrobial factors[10,11], though, nowadays an increasing heath concerns of consumers considering chemical preservatives as unhealthy[12]. For this reason, recently many researchers have been focused to create food products with less additives or utilizing natural compounds as additives to assure the quality and safety of food products[13-15]. Also, natural compounds as antimicrobial agent has less probability to develop Antimicrobial Resistance (AMR) on food borne pathogens. In this regard, probiotics, paraprobiotics and postbiotics as natural antimicrobial agents have gained more interest among the researchers[16,17]. Probiotics or Lactic Acid Bacteria (LAB) is one of the most investigated natural antimicrobial agents owing to their health well-being properties[18]. Despite the fact, the viable probiotic has some disadvantages; whereby to address this issue, paraprobiotics and postbiotics that were also from probiotics is an emerging concept to control the FBD and enhance human health. This review aimed to emphasize the advantageous effect of probiotics, paraprobiotics and postbiotics against food borne pathogens. The possible applications of paraprobiotics and postbiotics over probiotics were also discussed.
Food Borne Pathogens
FBD are often caused by contaminated food products. The causative agent for contaminated food products is illustrated in fig. 1. Bacteria and its toxins, viruses, fungi and parasites compounds are the major common reason for food-borne illness. The most common food-borne pathogens are listed in the Table 1. To treat the food-borne illness caused by food borne pathogens, antibiotics have been widely used. According to 2018 Food and Agricultural Organizational report 25 % of food borne pathogens exhibits resistance to one or more group of antibiotics. Therefore, most of the food-borne pathogens has resistance to antibiotics. Antibiotic resistance studies displays that most of the food-borne pathogens were resistant to minimum one antibiotic[19,20]. Therefore, overuse of antibiotics cause resistance in pathogenic genes in addition to their pathogenicity[21,22]. As a result, the food-borne pathogens act as a reservoir of resistance genes in addition of their pathogenicity[23]. In addition, impulsive usage of antibiotics is a major threat worldwide to the human population[22]. To overcome this drawback biological methods such as probiotic, postbiotic and parabiotic are a novel alternative approach.
| S. No | Food-borne bacteria | Sources | Incubation period | Symptoms | References |
|---|---|---|---|---|---|
| 1 | Salmonella species and its enterotoxins | Eggs, fruits, beef, sprouts, vegetables, pork, chicken, and nuts | 7-28 d | Vomiting, abdominal ache, constipation, rose spots, fever, nausea, headache, cough, chills, bloody stools, and fever | [55-60] |
| Ex: Salmonella typhi | |||||
| 2 | Staphylococcus and its enterotoxin | Meat, puddings, pastries, and sandwiches, | 1 d | Diarrhea, nausea, vomiting, and stomach cramps | |
| Ex: Staphylococcus aureus | |||||
| 3 | Clostridium and its neuro toxins | Meat (beef, poultry), food left for prolonged periods | 2 h-6 d, more often than not 12-36 h | Respiratory, loss of energy, paralysis vertigo, blurry vision, light reflex loss, complexity in swallowing, and dry mouth | |
| 5 | Bacillus cereus | Various foods especially rice and left overs, soups, sauces, and other cooked meals kept for extended period at ambient temperature. | 24 h | Vomiting, watery diarrhea, nausea and abdominal cramps | |
| 6 | Escherichia coli 0157:H7 | Contaminated water and food, uncooked beef, unpasteurized milk and juice, soft cheeses, infected people feces, cows, goats and sheep animal farms | 5-10 d | Hemolytic Uremic Syndrome (HUS) comprises less production of urine. Chronic diarrhea and stomach ache, no or low-grade fever, vomiting, urine is dark colored, and color change in lower eyelids and cheeks | |
| 7 | Listeria monocytogenes | Dairy products, soft cheese, raw fruits and vegetables, ready-to-eat foods; hot dogs and deli meats refrigerated foods such as pastries, meats, and smoked sea foods. | Days to several weeks | Fever, muscle ache, stiff neck, unsteadiness, and convulsions | |
| 8 | Shigella | Intake of contaminated food and water or via infected person | 5-7 d | Nausea, sudden stomach cramping, fever, diarrhea that might be bloody or mucus, and rectal tenesmus | |
| 9 | Norovirus | Ready-to-eat foods touched by infected food, intake of | 12-48 h | Vomiting, nausea, diarrhea and abdominal pain or cramp | |
| (NoV, SRSV, NLVs) | contaminated raw oysters or some other contaminated foods | ||||
| 10 | Hepatitis A and E | Intake of uncooked shellfish, drinking contaminated water. | 15-50 d | Dark urine or light-colored stools, jaundice and joint pain | |
| 11 | Toxoplasma gondii | Contaminated food or water consumption of uncooked meat or from infected animals and oocysts. | 10-23 d | Fever, inflamed lymph nodes, head and muscle ache | |
| 12 | Aspergillus species | Aflatoxins, ochratoxin, patulin | Certain stored fruits and vegetables, mushroom, wheat, maize, dairy products cereals and nuts | Vomiting abdominal pain, liver damage and cancer |
Table 1: The common food borne pathogens and its effect on human health.
Probiotics, Postbiotics and Paraprobiotics against Food-Borne Pathogens
Probiotic bacteria:
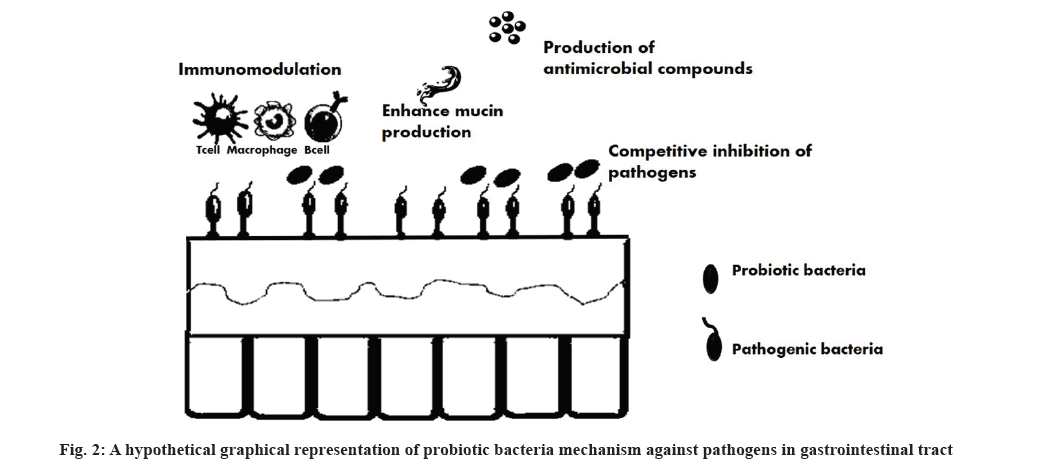
World Health Organization defined probiotics are live microorganism that confer a health benefit on the host and prevent diseases like inflammatory diseases, obesity, diabetes, food allergic reaction, lactose intolerance and food borne illness when administered in adequate amounts[24]. Probiotic bacteria eliminate the pathogenic organisms by the following mechanisms. Synthesis of antimicrobial compounds; immunomodulation; improvement of intestinal barrier performance; exclusion of pathogenic organisms by competitive mechanism. The existence of probiotics in gastro-intestinal tract interferes the pathogenic organism adhesion and undermining the pathogenicity as exhibited in fig. 2. Numerous investigations stated that probiotic bacteria have ability to reduce the propagation of pathogenic bacteria. Kariyawasam et al.[25] reported that Lactobacillus brevis act as antagonist against Escherichia coli O157:H4, Listeria monocytogenes, Salmonella enteritis and Staphylococcus aureus[25]. Another investigation revealed that five isolates of LAB from kimchi were inhibited the biofilm formation of Listeria monocytogenes[26]. As similar, Lactobacillus brevis identified from Italian cheese has investigated the Lactobacillus auto-aggregation and co-aggregation properties against Escherichia coli, Salmonella typhimurium, Staphylococcus aureus and Pseudomonas aeruginosa. The outcome of the study revealed that Lactobacillus strain has limited the proliferation of food borne pathogens by co-aggregation and auto-aggregation mechanisms[27]. A study conducted by Mahjoory et al.[28] stated that probiotic strain Limosilactobacillus fermentum fight against the aflatoxins producing organisms such as Aspergillus niger and Aspergillus flavus and it eliminate these organisms through auto-aggregation and co-aggregation mechanisms[28]. Also, probiotics was directly incorporated into the food products as bio-preservative in order to reduce or eliminate the foodborne pathogens in food products. Several studies were investigated the incorporation of probiotics into food products to hinder the proliferation of foodborne pathogens. In fresh cut pear researchers investigated the inter linkage between L. rhamnosus and pathogenic bacteria in fresh-cut pear and they found that L. rhamnosus reduces level of invasiveness of pathogenic bacteria[29]. Similarly in another investigations, Pediococcus acidilactici and Lactobacillus plantarum TN8 introduced into the food matrices of beef sausage substantially reduced the count of Enterobacteriaceae hence also extend the shelf life of sausage[30,31]. Likewise, the defensive effects of integration of probiotic organisms into food matrix were investigated in numerous studies. An extensive in vitro and in vivo research was conducted on probiotic strains such as Bifidobacterium brevis, L. paracasei, L. plantarum, L. delbrueckii, Clostridium butyricum, L. rhamnosus GG, L. gasseri, L. helveticus, L. acidophilus, L. reuteri ATCC 55730, Bacillus thermophilum RBL67, Enterococcus faecium, L. crispatus, L. gallinarum, L. rhamnosus J10-L and L. casei Q8-L and the outcome of the studies showed that probiotics have reduced the effect of major food borne pathogens namely E. coli O157:H7, Salmonella species, Campylobacter jejuni, Listeria monocytogenes and Shigella species[32-34]. In addition, animal studies displays that certain probiotics has antiviral effect by blocking the viral adhesion, replication and antiviral compound synthesis[35,36].
Numerous clinical evidences have confirmed that probiotic strains reduce the risk associated with food borne pathogens[37,38]. Even if the utilization of probiotics is a promising approach, there are some concerns for the usage of probiotics such as transfer of virulence gene, emergence of bacteremia in immuno compromised patients, development of antibiotic resistance, also short durability and stability have hinder their prospective application in the pharmaceuticals and food sectors[39-41]. To overcome these issues, paraprobiotics and postbiotics the other form of probiotics are being investigated against food-borne pathogens owing to the enhanced health and well-being properties of host[42].
Postbiotics and paraprobiotics:
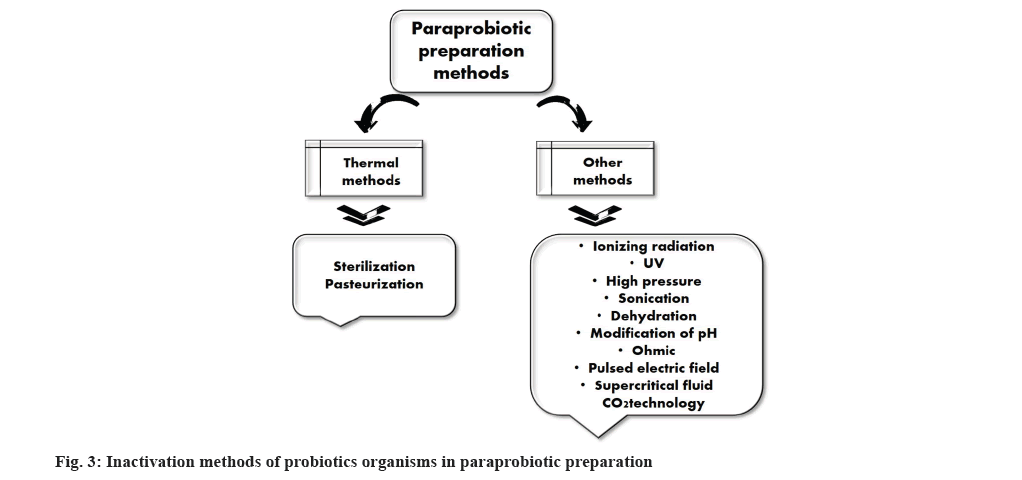
Paraprobiotics are also called dead cells or non-viable probiotics or ghost-probiotics which provides health benefits to animals and humans when administered in sufficient quantity. The probiotics inactivation can be achieved by different methods as represented in fig. 3. Paraprobiotics entirely lost their viability after being exposed to elements that change microbial cell structures, such as mechanical damage to the envelope of cell, key enzyme’s inactivation and DNA filament breaks[43]. Hence, the paraprobiotics are the inactivated probiotic bacterial cells contain cell constituents include teichoic acid, surface proteins, or crude extracts of microbial cells holds complex composition of chemicals[44]. However, several studies have stated that paraprobiotics display a tremendous health benefit characteristics more than the probiotic bacteria[45,46]. A very modest investigation was conducted on paraprobiotics against food-borne pathogens. Tareb et al.[47] examined the activity of heat inactivated or paraprobiotics L. farciminis CNCM-I-3699 and L. rhamnosus CNCM-I-3698 activity against the poultry based food borne pathogen Camphylobacter jeujeni and found that paraprobiotics strongly inhibited the adhesion of Camphylobacter jeujeni in the intestinal mucin[47]. In a mice model of Helicobacter pylori infection, the paraprobiotic Lactobacillus johnsonii has decreased the cell count of H. pylori after the recurrent consumption of heat killed L. johnsonii strain[48]. In vitro investigation of five viable and non-viable strains Levilactobacillus brevis O22, Levilactobacillus brevis O24, Lacticaseibacillus casei O12, Lacticaseibacillus casei O16, and Lactiplantibacillus plantarum O20 were tested against pathogens L. monocytogenes 15313 and S. aureus 25923. The outcome of the investigation showed that the five viable and non-viable LAB strains were preventing the colonization of pathogenic strains by coaggregation. Also, non-viable LAB strains have high activity against the pathogen than viable LAB strains[49]. Similarly, heat inactivated LAB strains such as L. fermentum,Enterococcus faecium,L. acidophilus,andL. plantarum reduced the Salmonella infection in a mice model. The authors reported that antagonist effect of heat inactivated LAB strains might attributed to the presence exopolysaccharides and lipoteichoic acid[50].
Postbiotic refers to inactive microbe’s cell components, or metabolites that are released through fermentation by probiotic microorganisms. In most of the studies, postbiotics are referred as filtered Cell Free Supernatant (CFS) which is produced from the fermented probiotic culture media, is where postbiotics are derived in the majority of research[51]. Postbiotics is a mixture of bioactive substances instead of single purified compounds; the postbiotics are the intricate composition of metabolic end products produced by probiotics in CFS included secreted proteins, short chain fatty acids, organic acids, peptides, vitamins, enzymes, secreted biosurfactants and amino acids, etc.,[51,52]. The mechanisms of postbiotics are not clearly understood. However, postbiotics might modulate and provoke the immunological response of host.
Postbiotics antimicrobial mechanisms majorly depend on the compounds secreted by probiotic bacteria. Therefore, aforesaid postbiotic compounds might interpret the enzyme’s structure and activity, electron transport chain, membrane degradation of pathogenic organisms, prohibition of macromolecule synthesis and create unfavorable condition for survival of pathogenic organisms through reduce the pH of the environment[54]. Due to the advantages of postbiotics, researches on postbiotics against food borne pathogens are increasing. Moreover, many in vitro and in vivo studies conducted by researchers showed that antimicrobial activity of postbiotics against pathogenic organism. The studies which used postbiotics against foodborne pathogens are listed in Table 1 and Table 2.
| Postbiotic solution obtained probiotic bacteria | Target pathogenic bacteria | References |
|---|---|---|
| Lactobacillus plantarum | Klebsiella pneumonia, Cronobacter sakazakii, Vibrio parahaemolyticus, Pseudomonas aeruginosa, Salmonella enterica, Staphylococcus mutans, S. aureus, Listeria monocytogenes | [61-66] |
| Pediococous pentosaceus 4I1 | Bacillus subtilis, S. enterica, S. aureus, E. coli O157:H7, L. Monocytogenes, | |
| L. acidophilus, L. salivarius | E. coli | |
| Lactobacillus delbrueckii subsp. | Clostidium perfringens | |
| L. rhamnosus | ||
| P. acidilactici | ||
| L. fermentum | ||
| L. casei 431 | S. aureus | |
| L. acidophilus LA5 | ||
| Leuconostoc mesenteroides | E. coli, Vibrio parahaemolyticus and Pseudomonas aeruginosa | |
| Pediococcus acidilactici | L. monocytogenes, Salmonella typhirium, | |
| Escherichia coli O157:H7, | ||
| Lactic acid bacteria (Pediococcus acidilactici, Streptococcus thermophilus, Leuconostoc mesenteroides) | Escherichia coli and Salmonella paratyphi A |
Table 2: Antimicrobial activity of postbiotics against food borne pathogens.
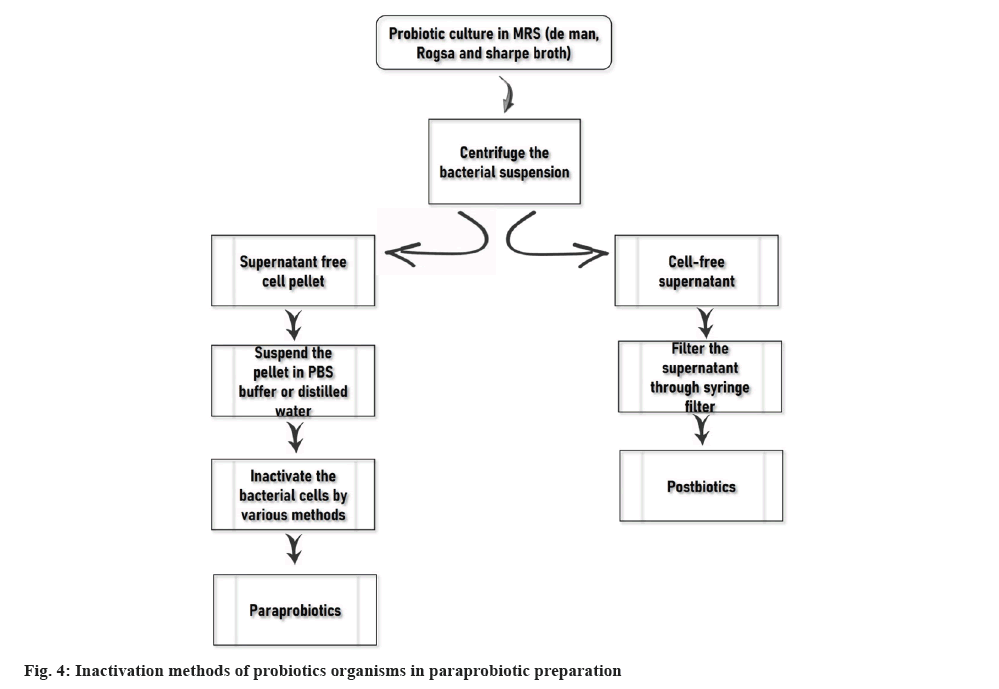
Paraprobiotics and postbiotics have various benefits over probiotics i.e. pure form of their availability, easy storage and synthesis, precise mechanism of action and no possibility to antibiotic resistance genes transfer. The process flowchart for the synthesis of paraprobiotics and postbiotics is represented in fig. 4[55]. The use paraprobiotics and postbiotics allow overtaking the several disadvantages generated by probiotics. Therefore, the unique characteristics of postbiotic and paraprobiotics have drawn attention in other research areas such as obesity, hypertension, cancer, longer storage stability, and ability to trigger several systems governing inflammation, cardiovascular disease and oxidative stress. Similar to how human health has drawn attention, postbiotic and paraprobiotics played an important role in animal health as well[52,53]. Also, postbiotics and paraprobiotics uncover new prospects in the pharmaceutical and food industry.
Future Remarks
Many food-borne pathogens are resistant to antibiotics, which are used to treat the FBD. Meanwhile, another therapeutic strategy is being examined to minimize the up-surging antibiotic resistant pathogens. Probiotics, paraprobiotics and postbiotics are the promising alternative natural antibiotic therapy against food-borne pathogens. On the other hand, probiotic provides numerous health benefits to the host, but the above-mentioned drawbacks are the major limit for the probiotic organisms. Therefore, paraprobiotics and postbiotics would be a great substitute for antibiotic therapy, but efficiency of paraprobiotics and postbiotics relies on the efficiency of probiotic strains. On the contrary, lack of information about the paraprobiotics and postbiotics in in vivo, in vitro and clinical studies restrict the therapeutic and industrial application. Hence, it is significant to perform investigation to understand effects of paraprobiotics and postbiotics on human health and their gut microbiome interaction, also their incorporation into food products prevent the food spoilage by pathogens which are the significant cause of FBD. The deeper examination of paraprobiotics and postbiotics by the researcher will aids to develop novel prospect for the production of healthier, sustainable, natural and safer products.
Author’s contribution:
Ragavi Baskar and Vishnupriya Subramaniyan contributed equally to this work.
Acknowledgement:
We would like to thank SRM Institute of Science and Technology for their help and cordial support of our study.
Conflict of interest:
The author declares no conflict of interest.
References
- Hutt PB. A history of government regulation of adulteration and misbranding of medical devices. Food Drug Cosm L J 1989;44(2):99-117.
- Mohammad AM, Chowdhury T, Biswas B, Absar N. Food poisoning and intoxication: A global leading concern for human health. Food Safety and Preservation 2018;307-52.
- Majumdar A, Pradhan N, Sadasivan J, Acharya A, Ojha N, Babu S, et al. Food degradation and foodborne diseases: A microbial approach. Microbial Contamination and Food Degradation. 2018;109-48.
- World Health Organization. WHO estimates of the global burden of foodborne diseases. World Health Organization 2015.
- Amit SK, Uddin MdM, Rahman R, Islam SMR, Khan MS. A review on mechanisms and commercial aspects of food preservation and processing. Agric Food Secur 2017;6(1):51.
- Anitha DPM, Sellamuthu PS. Microencapsulation of probiotics in finger millet milk complex to improve encapsulation efficiency and viability. Food Sci Technol Int 2021;28(3):216-32.
[Crossref] [Google Scholar] [PubMed]
- Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, et al. Food-related illness and death in the United States. Emerg Infect Dis 1999;5(5):607-25.
[Crossref] [Google Scholar] [PubMed]
- King JC, Black RE, Doyle MP, Fritsche KL, Halbrook BH, Levander OA, et al. Foodborne illnesses and nutritional status: A statement from an American society for nutritional sciences working group. J Nutr 2000;130(10):2613-7.
[Crossref] [Google Scholar] [PubMed]
- Dewey-Mattia D, Manikonda K, Hall AJ, Wise ME, Crowe SJ. Surveillance for foodborne disease outbreaks-United States, 2009-2015. MMWR Surveill Summ 2018;67(10):1-11.
[Crossref] [Google Scholar] [PubMed]
- Jayaraman L, Sellamuthu PS, Siva DP, Aravindan RS, Vellaichamy V, Anitha PM. In vitro antibacterial, antioxidant and mechanism of antifungal activities of selected Indian traditional plants against human oral microbes. Indian J Pharm Sci 2021;83(5):1024-32.
- Singh VP. Recent approaches in food bio-preservation-a review. Open Vet J 2018;8(1):104-11.
[Crossref] [Google Scholar] [PubMed]
- Chang Y, Meng J, Di Q, Lou S, Feng X, Zhang D. Effect of Lactobacillus rhamnosus on intestinal mucosal barrier function in mice with post-infectious irritable bowel syndrome. Indian J Pharm Sci 2020;82(6):1040-3.
- Bearth A, Cousin ME, Siegrist M. The consumer’s perception of artificial food additives: Influences on acceptance, risk and benefit perception. Food Qual Prefer 2014;38:14-23.
- Kumar S, Mukherjee A, Dutta J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci Technol 2020;97:196-209.
- Guimaraes JT, Balthazar CF, Silva R, Rocha RS, Graça JS, Esmerino EA, et al. Impact of probiotics and prebiotics on food texture. Curr Opin Food Sci 2020;33:38-44.
- Koirala S, Anal AK. Probiotics-based foods and beverages as future foods and their overall safety and regulatory claims. Future Foods 2021;3:100013.
- Martyniak A, Medynska-Przęczek A, Wędrychowicz A, Skoczen S, Tomasik PJ. Prebiotics, probiotics, synbiotics, paraprobiotics and postbiotic compounds in IBD. Biomolecules 2021;11(12):1903.
[Crossref] [Google Scholar] [PubMed]
- Reid G. The scientific basis for probiotic strains of Lactobacillus. Appl Environ Microbiol 1999;65(9):3763-6.
[Crossref] [Google Scholar] [PubMed]
- Mensah SE, Koudande OD, Sanders P, Laurentie M, Mensah GA, Abiola FA. Antimicrobial residues in foods of animal origin in Africa: Public health risks. Rev Sci Tech 2014;33(3):987-96.
[Google Scholar] [PubMed]
- Fair RJ, Tor Y. Antibiotics and bacterial resistance in the 21st century. Perspect Medicin Chem 2014;6:25-64.
[Crossref] [Google Scholar] [PubMed]
- Shafipour Yordshahi A, Moradi M, Tajik H, Molaei R. Design and preparation of antimicrobial meat wrapping nanopaper with bacterial cellulose and postbiotics of lactic acid bacteria. Int J Food Microbiol 2020;321:108561.
[Crossref] [Google Scholar] [PubMed]
- Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog Glob Health 2015;109(7):309-18.
[Crossref] [Google Scholar] [PubMed]
- De Moreno de Leblanc A, Del Carmen S, Zurita-Turk M, Santos Rocha C, Van De Guchte M, Azevedo V, et al. Importance of IL-10 modulation by probiotic microorganisms in gastrointestinal inflammatory diseases. ISRN Gastroenterol 2011;2011:e892971.
[Crossref] [Google Scholar] [PubMed]
- Subramaniyan V, Gurumurthy K. Diversity of probiotic adhesion genes in the gastrointestinal tract of goats. J Cell Biochem 2019;120(8):12422-8.
[Crossref] [Google Scholar] [PubMed]
- Kariyawasam KMGMM, Yang SJ, Lee NK, Paik HD. Probiotic properties of Lactobacillus brevis KU200019 and synergistic activity with fructooligosaccharides in antagonistic activity against foodborne pathogens. Food Sci Anim Resour 2020;40(2):297-310.
[Crossref] [Google Scholar] [PubMed]
- Hossain MI, Mizan MFR, Ashrafudoulla M, Nahar S, Joo HJ, Jahid IK, et al. Inhibitory effects of probiotic potential lactic acid bacteria isolated from kimchi against Listeria monocytogenes biofilm on lettuce, stainless-steel surfaces, and MBECTM biofilm device. LWT. 2020;118:108864.
- Kavitha S, Harikrishnan A, Jeevaratnam K. Characterization and evaluation of antibacterial efficacy of a novel antibiotic-type compound from a probiotic strain Lactobacillus plantarum KJB23 against food-borne pathogens. LWT 2020;118:108759.
- Mahjoory Y, Mohammadi R, Hejazi MA, Nami Y. Antifungal activity of potential probiotic Limosilactobacillus fermentum strains and their role against toxigenic aflatoxin-producing aspergilli. Sci Rep 2023;13(1):388.
[Crossref] [Google Scholar] [PubMed]
- Iglesias MB, Echeverría G, Vinas I, Lopez ML, Abadias M. Biopreservation of fresh-cut pear using Lactobacillus rhamnosus GG and effect on quality and volatile compounds. LWT 2018;87:581-8.
- Haraguchi Y, Goto M, Kuda T, Fukunaga M, Shikano A, Takahashi H, et al. Inhibitory effect of Lactobacillus plantarumTennozu-SU2 andLactococcus lactis subsp. lactis BF1 on Salmonella Typhimuriumand Listeria monocytogenesduring and post fermentation of soymilk. LWT 2019;102:379-84.
- Ben Slima S, Ktari N, Triki M, Trabelsi I, Abdeslam A, Moussa H, et al. Effects of probiotic strains, Lactobacillus plantarumTN8 and Pediococcus acidilactici, on microbiological and physico-chemical characteristics of beef sausages. LWT 2018;92:195-203.
- McFarland LV, Evans CT, Goldstein EJ. Strain-specificity and disease-specificity of probiotic efficacy: A systematic review and meta-analysis. Front Med 2018;5:124.
[Crossref] [Google Scholar] [PubMed]
- Kandasamy S, Chattha KS, Vlasova AN, Rajashekara G, Saif LJ. Lactobacilli and Bifidobacteria enhance mucosal B cell responses and differentially modulate systemic antibody responses to an oral human rotavirus vaccine in a neonatal gnotobiotic pig disease model. Gut Microbes 2014;5(5):639-51.
[Crossref] [Google Scholar] [PubMed]
- Kreuzer S, Machnowska P, Abmus J, Sieber M, Pieper R, Schmidt MF, et al. Feeding of the probiotic bacterium Enterococcus faeciumNCIMB 10415 differentially affects shedding of enteric viruses in pigs. Vet Res 2012;43(1):58.
[Crossref] [Google Scholar] [PubMed]
- Wacoo AP, Mukisa IM, Meeme R, Byakika S, Wendiro D, Sybesma W, et al. Probiotic enrichment and reduction of aflatoxins in a traditional African maize-based fermented food. Nutrients 2019;11(2):265.
[Crossref] [Google Scholar] [PubMed]
- Sangave PC. Pathogenesis and drug resistance profile of food-borne pathogens. Model Organisms for Microbial Pathogenesis, Biofilm Formation and Antimicrobial Drug Discovery. 2020;349-77.
- Fenster K, Freeburg B, Hollard C, Wong C, Ronhave Laursen R, Ouwehand AC. The production and delivery of probiotics: A review of a practical approach. Microorganisms 2019;7(3):83.
[Crossref] [Google Scholar] [PubMed]
- Sadiq FA, Yan B, Tian F, Zhao J, Zhang H, Chen W. Lactic acid bacteria as antifungal and anti-mycotoxigenic agents: A comprehensive review. Compr Rev Food Sci Food Saf 2019;18(5):1403-36.
[Crossref] [Google Scholar] [PubMed]
- Yordshahi AS, Moradi M, Tajik H, Molaei R. Design and preparation of antimicrobial meat wrapping nanopaper with bacterial cellulose and postbiotics of lactic acid bacteria. Int J Food Microbiol 2020;321:108561.
[Crossref] [Google Scholar] [PubMed]
- Deshpande G, Athalye-Jape G, Patole S. Para-probiotics for preterm neonates-the next frontier. Nutrients 2018;10(7):871.
[Crossref] [Google Scholar] [PubMed]
- Barros CP, Guimaraes J, Esmerino E, Duarte M, Silva MC, Silva R, et al. Paraprobiotics and postbiotics: concepts and potential applications in dairy products. Curr Opin Food Sci 2020;32:1-8.
- Kanauchi O, Andoh A, AbuBakar S, Yamamoto N. Probiotics and paraprobiotics in viral infection: Clinical application and effects on the innate and acquired immune systems. Curr Pharm Des 2018;24(6):710-7.
[Crossref] [Google Scholar] [PubMed]
- de Almada CN, Almada CN, Martinez RC, Sant'Ana AS. Paraprobiotics: Evidences on their ability to modify biological responses, inactivation methods and perspectives on their application in foods. Trends Food Sci Technol 2016;58:96-114.
- Teame T, Wang A, Xie M, Zhang Z, Yang Y, Ding Q, et al. Paraprobiotics and postbiotics of probiotic Lactobacilli, their positive effects on the host and action mechanisms: A review. Front Nutr 2020;7:570344.
[Crossref] [Google Scholar] [PubMed]
- Jang HJ, Song MW, Lee NK, Paik HD. Antioxidant effects of live and heat-killed probiotic Lactobacillus plantarum Ln1 isolated from kimchi. J Food Sci Technol 2018;55(8):3174-80.
[Crossref] [Google Scholar] [PubMed]
- Hamad GM, Abdelmotilib NM, Darwish AMG, Zeitoun AM. Commercial probiotic cell-free supernatants for inhibition of Clostridium perfringens poultry meat infection in Egypt. Anaerobe 2020;62:102181.
[Crossref] [Google Scholar] [PubMed]
- Tareb R, Bernardeau M, Gueguen M, Vernoux JP. In vitro characterization of aggregation and adhesion properties of viable and heat-killed forms of two probiotic Lactobacillus strains and interaction with foodborne zoonotic bacteria, especially Campylobacter jejuni. J Med Microbiol 2013;62(4):637-49.
[Crossref] [Google Scholar] [PubMed]
- Aiba Y, Ishikawa H, Tokunaga M, Komatsu Y. Anti-Helicobacter pylori activity of non-living, heat-killed form of lactobacilli including Lactobacillus johnsoniiNo.1088. FEMS Microbiol Lett 2017;364(11):fnx102.
[Crossref] [Google Scholar] [PubMed]
- Karbowiak M, Gałek M, Szydłowska A, Zielinska D. The influence of the degree of thermal inactivation of probiotic lactic acid bacteria and their postbiotics on aggregation and adhesion inhibition of selected pathogens. Pathogens 2022;11(11):1260.
[Crossref] [Google Scholar] [PubMed]
- Chen CY, Tsen HY, Lin CL, Lin CK, Chuang LT, Chen CS, et al. Enhancement of the immune response against Salmonella infection of mice by heat-killed multispecies combinations of lactic acid bacteria. J Med Microbiol 2013;62(Pt 11):1657-64.
[Crossref] [Google Scholar] [PubMed]
- Mantziari A, Salminen S, Szajewska H, Malagón-Rojas JN. Postbiotics against pathogens commonly involved in pediatric infectious diseases. Microorganisms 2020;8(10):1510.
[Crossref] [Google Scholar] [PubMed]
- Nataraj BH, Ali SA, Behare PV, Yadav H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb Cell Fact 2020;19(1):168.
[Crossref] [Google Scholar] [PubMed]
- Aghebati-Maleki L, Hasannezhad P, Abbasi A, Khani N. Antibacterial, antiviral, antioxidant, and anticancer activities of postbiotics: A review of mechanisms and therapeutic perspectives. Biointerface Res Appl Chem 2021;12(2):2629-45.
- Hsieh FC, Lan CCE, Huang TY, Chen KW, Chai CY, Chen WT, et al. Heat-killed and live Lactobacillus reuteri GMNL-263 exhibit similar effects on improving metabolic functions in high-fat diet-induced obese rats. Food Funct 2016;7(5):2374-88.
[Crossref] [Google Scholar] [PubMed]
- Bintsis T. Foodborne pathogens. AIMS Microbiol 2017;3(3):529-63.
[Crossref] [Google Scholar] [PubMed]
- Ehuwa O, Jaiswal AK, Jaiswal S. Salmonella, food safety and food handling practices. Foods 2021;10(5):907.
[Crossref] [Google Scholar] [PubMed]
- Hedberg CW, Osterholm MT. Outbreaks of food-borne and waterborne viral gastroenteritis. Clin Microbiol Rev 1993;6(3):199-210.
[Crossref] [Google Scholar] [PubMed]
- Dorny P, Praet N, Deckers N, Gabriel S. Emerging food-borne parasites. Vet Parasitol 2009;163(3):196-206.
[Crossref] [Google Scholar] [PubMed]
- Thambugala KM, Daranagama DA, Phillips AJL, Kannangara SD, Promputtha I. Fungi vs. fungi in biocontrol: An overview of fungal antagonists applied against fungal plant pathogens. Front Cell Infect Microbiol 2020;10:604923.
[Crossref] [Google Scholar] [PubMed]
- Navale V, Vamkudoth KR, Ajmera S, Dhuri V. Aspergillus derived mycotoxins in food and the environment: Prevalence, detection, and toxicity. Toxicol Rep 2021;8:1008-30.
[Crossref] [Google Scholar] [PubMed]
- Incili GK, Akgol M, Karatepe P, Tekin A, Kanmaz H, Kaya B, et al. Whole-cell postbiotics: An innovative approach for extending the shelf life and controlling major foodborne pathogens in chicken breast fillets. Food Bioprocess Technol 2023;16:1502-24.
- Yilmaz N, Ozogul F, Moradi M, Fadiloglu EE, Simat V, Rocha JM. Reduction of biogenic amines formation by foodborne pathogens using postbiotics in lysine-decarboxylase broth. J Biotechnol 2022;358:118-27.
- Toushik SH, Park JH, Kim K, Ashrafudoulla M, Ulrich MS, Mizan MF, et al. Antibiofilm efficacy of Leuconostoc mesenteroides J.27-derived postbiotic and food-grade essential oils against Vibrio parahaemolyticus, Pseudomonas aeruginosa, and Escherichia coli alone and in combination, and their application as a green preservative in the seafood industry. Food Res Int 2022;156:111163.
[Crossref] [Google Scholar] [PubMed]
- Moradi M, Mardani K, Tajik H. Characterization and application of postbiotics of Lactobacillus spp. on Listeria monocytogenes in vitro and in food models. LWT 2019;111:457-64. [Crossref]
- Ołdak A, Zielinska D, Łepecka A, Długosz E, Kołozyn-Krajewska D. Lactobacillus plantarum strains isolated from polish regional cheeses exhibit anti-staphylococcal activity and selected probiotic properties. Probiotics Antimicrob Proteins 2020;12(3):1025-38.
[Crossref] [Google Scholar] [PubMed]
- Bajpai VK, Han JH, Rather IA, Park C, Lim J, Paek WK, et al. Characterization and antibacterial potential of lactic acid bacterium Pediococcus pentosaceus 4I1 isolated from freshwater fish zacco koreanus. Front Microbiol 2016;7:2037.
[Crossref] [Google Scholar] [PubMed]